Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity
- PMID: 17114296
- PMCID: PMC1643842
- DOI: 10.1073/pnas.0602235103
Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity
Abstract
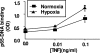
Hypoxia is a feature of the microenvironment of a growing tumor. The transcription factor NFkappaB is activated in hypoxia, an event that has significant implications for tumor progression. Here, we demonstrate that hypoxia activates NFkappaB through a pathway involving activation of IkappaB kinase-beta (IKKbeta) leading to phosphorylation-dependent degradation of IkappaBalpha and liberation of NFkappaB. Furthermore, through increasing the pool and/or activation potential of IKKbeta, hypoxia amplifies cellular sensitivity to stimulation with TNFalpha. Within its activation loop, IKKbeta contains an evolutionarily conserved LxxLAP consensus motif for hydroxylation by prolyl hydroxylases (PHDs). Mimicking hypoxia by treatment of cells with siRNA against PHD-1 or PHD-2 or the pan-prolyl hydroxylase inhibitor DMOG results in NFkappaB activation. Conversely, overexpression of PHD-1 decreases cytokine-stimulated NFkappaB reporter activity, further suggesting a repressive role for PHD-1 in controlling the activity of NFkappaB. Hypoxia increases both the expression and activity of IKKbeta, and site-directed mutagenesis of the proline residue (P191A) of the putative IKKbeta hydroxylation site results in a loss of hypoxic inducibility. Thus, we hypothesize that hypoxia releases repression of NFkappaB activity through decreased PHD-dependent hydroxylation of IKKbeta, an event that may contribute to tumor development and progression through amplification of tumorigenic signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


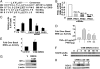

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

