Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts
- PMID: 17114352
- PMCID: PMC1693946
- DOI: 10.1105/tpc.106.045641
Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts
Abstract
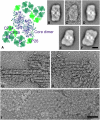
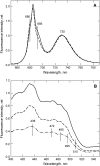
The photosystem II (PSII) light-harvesting antenna in higher plants contains a number of highly conserved gene products whose function is unknown. Arabidopsis thaliana plants depleted of one of these, the CP24 light-harvesting complex, have been analyzed. CP24-deficient plants showed a decrease in light-limited photosynthetic rate and growth, but the pigment and protein content of the thylakoid membranes were otherwise almost unchanged. However, there was a major change in the macroorganization of PSII within these membranes; electron microscopy and image analysis revealed the complete absence of the C(2)S(2)M(2) light-harvesting complex II (LHCII)/PSII supercomplex predominant in wild-type plants. Instead, only C(2)S(2) supercomplexes, which are deficient in the LHCIIb M-trimers, were found. Spectroscopic analysis confirmed the disruption of the wild-type macroorganization of PSII. It was found that the functions of the PSII antenna were disturbed: connectivity between PSII centers was reduced, and maximum photochemical yield was lowered; rapidly reversible nonphotochemical quenching was inhibited; and the state transitions were altered kinetically. CP24 is therefore an important factor in determining the structure and function of the PSII light-harvesting antenna, providing the linker for association of the M-trimer into the PSII complex, allowing a specific macroorganization that is necessary both for maximum quantum efficiency and for photoprotective dissipation of excess excitation energy.
Figures








References
-
- Allen, J.F. (1992). Protein-phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098 275–335. - PubMed
-
- Allen, J.F., and Forsberg, J. (2001). Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6 317–326. - PubMed
-
- Anderson, J.M., Chow, W.S., and Park, Y.I. (1995). The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 46 129–139. - PubMed
-
- Andersson, J., Wentworth, M., Walters, R.G., Howard, C.A., Ruban, A.V., Horton, P., and Jansson, S. (2003). Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II - Effects on photosynthesis, grana stacking and fitness. Plant J. 35 350–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

