Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor
- PMID: 17114443
- PMCID: PMC2819292
- DOI: 10.4049/jimmunol.177.11.7723
Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor
Abstract
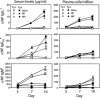
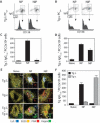
Long-lived plasma cells (PCs) and memory B cells (B(mem)) constitute the cellular components of enduring humoral immunity, whereas short-lived PCs that rapidly produce Ig correspond to the host's need for immediate protection against pathogens. In this study we show that the innate affinity of the BCR for Ag imprints upon naive B cells their differentiation fate to become short- or long-lived PCs and B(mem). Using BCR transgenic mice with varying affinities for Ag, naive B cells with high affinity lose their capacity to form germinal centers (GCs), develop neither B(mem) nor long-lived PCs, and are destined to a short-lived PC fate. Moderate affinity interactions result in hastened GC responses, and differentiation to long-lived PCs, but B(mem) remain extinct. In contrast, lower affinity interactions show tempered GCs, producing B(mem) and affinity-matured, long-lived PCs. Thus, a continuum of elementary to comprehensive humoral immune responses exists that is controlled by inherent BCR affinity.
Figures



Similar articles
-
Cbl Ubiquitin Ligases Control B Cell Exit from the Germinal-Center Reaction.Immunity. 2018 Mar 20;48(3):530-541.e6. doi: 10.1016/j.immuni.2018.03.006. Immunity. 2018. PMID: 29562201
-
Positive Selection in the Light Zone of Germinal Centers.Front Immunol. 2021 Mar 31;12:661678. doi: 10.3389/fimmu.2021.661678. eCollection 2021. Front Immunol. 2021. PMID: 33868314 Free PMC article. Review.
-
Determinations of B cell fate in immunity and autoimmunity.Curr Dir Autoimmun. 2005;8:1-24. doi: 10.1159/000082084. Curr Dir Autoimmun. 2005. PMID: 15564715 Review.
-
Affinity of antigen encounter and other early B-cell signals determine B-cell fate.Curr Opin Immunol. 2007 Jun;19(3):275-80. doi: 10.1016/j.coi.2007.04.009. Epub 2007 Apr 12. Curr Opin Immunol. 2007. PMID: 17433651 Free PMC article. Review.
-
Determining germinal centre B cell fate.Trends Immunol. 2012 Jun;33(6):281-8. doi: 10.1016/j.it.2012.04.003. Epub 2012 May 16. Trends Immunol. 2012. PMID: 22595532 Review.
Cited by
-
Origin and Function of Circulating Plasmablasts during Acute Viral Infections.Front Immunol. 2012 Apr 17;3:78. doi: 10.3389/fimmu.2012.00078. eCollection 2012. Front Immunol. 2012. PMID: 22566959 Free PMC article.
-
Optimality of mutation and selection in germinal centers.PLoS Comput Biol. 2010 Jun 3;6(6):e1000800. doi: 10.1371/journal.pcbi.1000800. PLoS Comput Biol. 2010. PMID: 20532164 Free PMC article.
-
Anti-B-Cell Therapies in Autoimmune Neurological Diseases: Rationale and Efficacy Trials.Neurotherapeutics. 2016 Jan;13(1):20-33. doi: 10.1007/s13311-015-0402-6. Neurotherapeutics. 2016. PMID: 26566961 Free PMC article. Review.
-
Single-B cell analysis correlates high-lactate secretion with stress and increased apoptosis.Sci Rep. 2024 Apr 12;14(1):8507. doi: 10.1038/s41598-024-58868-0. Sci Rep. 2024. PMID: 38605071 Free PMC article.
-
Germinal center B cell initiation, GC maturation, and the coevolution of its stromal cell niches.Immunol Rev. 2019 Mar;288(1):10-27. doi: 10.1111/imr.12731. Immunol Rev. 2019. PMID: 30874342 Free PMC article. Review.
References
-
- MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. - PubMed
-
- Kelsoe G. In situ studies of the germinal center reaction. Adv. Immunol. 1995;60:267–288. - PubMed
-
- Gray D, Siepmann K, van Essen D, Poudrier J, Wykes M, Jainandunsing S, Bergthorsdottir S, Dullforce P. B-T lymphocyte interactions in the generation and survival of memory cells. Immunol. Rev. 1996;150:45–61. - PubMed
-
- Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J. Immunol. 1998;161:5373–5381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

