Wnt/beta-catenin signaling regulates vertebrate limb regeneration
- PMID: 17114576
- PMCID: PMC1686599
- DOI: 10.1101/gad.1475106
Wnt/beta-catenin signaling regulates vertebrate limb regeneration
Abstract
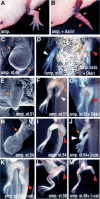
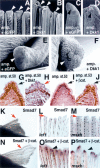
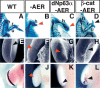
The cellular and molecular bases allowing tissue regeneration are not well understood. By performing gain- and loss-of-function experiments of specific members of the Wnt pathway during appendage regeneration, we demonstrate that this pathway is not only necessary for regeneration to occur, but it is also able to promote regeneration in axolotl, Xenopus, and zebrafish. Furthermore, we show that changes in the spatiotemporal distribution of beta-catenin in the developing chick embryo elicit apical ectodermal ridge and limb regeneration in an organism previously thought not to regenerate. Our studies may provide valuable insights toward a better understanding of adult tissue regeneration.
Figures

References
-
- Ahn, K., Mishina, Y., Hanks, M.C., Behringer, R.R., Crenshaw, E.B., III BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development. 2001;128:4449–4461. - PubMed
-
- Akimenko, M.A., Mari-Beffa, M., Becerra, J., Geraudie, J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 2003;226:190–201. - PubMed
-
- Bakkers, J., Hild, M., Kramer, C., Furutani-Seiki, M., Hammerschmidt, M. Zebrafish ΔNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev. Cell. 2002;2:617–627. - PubMed
-
- Bode, H.R. Head regeneration in Hydra. Dev. Dyn. 2003;226:225–236. - PubMed
-
- Brockes, J.P., Kumar, A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell Biol. 2002;3:566–574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
