Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport
- PMID: 17114598
- PMCID: PMC1800364
- DOI: 10.1128/EC.00318-06
Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport
Abstract
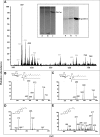
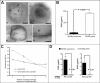
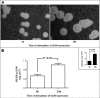
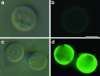
The mechanisms by which macromolecules are transported through the cell wall of fungi are not known. A central question in the biology of Cryptococcus neoformans, the causative agent of cryptococcosis, is the mechanism by which capsular polysaccharide synthesized inside the cell is exported to the extracellular environment for capsule assembly and release. We demonstrate that C. neoformans produces extracellular vesicles during in vitro growth and animal infection. Vesicular compartments, which are transferred to the extracellular space by cell wall passage, contain glucuronoxylomannan (GXM), a component of the cryptococcal capsule, and key lipids, such as glucosylceramide and sterols. A correlation between GXM-containing vesicles and capsule expression was observed. The results imply a novel mechanism for the release of the major virulence factor of C. neoformans whereby polysaccharide packaged in lipid vesicles crosses the cell wall and the capsule network to reach the extracellular environment.
Figures


References
-
- Barreto-Bergter, E., M. R. Pinto, and M. L. Rodrigues. 2004. Structure and biological functions of fungal cerebrosides. An. Acad. Bras. Cienc. 76:67-84. - PubMed
-
- Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. - PubMed
-
- Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

