A-to-I RNA editing and human disease
- PMID: 17114938
- PMCID: PMC2947206
- DOI: 10.4161/rna.3.1.2495
A-to-I RNA editing and human disease
Abstract
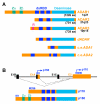
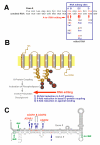
The post-transcriptional modification of mammalian transcripts by A-to-I RNA editing has been recognized as an important mechanism for the generation of molecular diversity and also regulates protein function through recoding of genomic information. As the molecular players of editing are characterized and an increasing number of genes become identified that are subject to A-to-I modification, the potential impact of editing on the etiology or progression of human diseases is realized. Here we review the recent knowledge on where disturbances in A-to-I RNA editing have been correlated with human disease phenotypes.
Figures


References
-
- Johnson JM, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. - PubMed
-
- Keegan LP, Gallo A, O’Connell MA. The many roles of an RNA editor. Nat Rev Genet. 2001;2:869–78. - PubMed
-
- Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu Rev Genet. 2000;34:499–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
