Laboratory diagnosis of human seasonal and pandemic influenza virus infection
- PMID: 17115952
- PMCID: PMC7168388
- DOI: 10.5694/j.1326-5377.2006.tb00707.x
Laboratory diagnosis of human seasonal and pandemic influenza virus infection
Abstract
Laboratory diagnosis is important to distinguish influenza from other respiratory virus infections. It will be especially important in detecting the first cases of pandemic influenza. Good quality respiratory tract sampling is needed to maximise diagnostic yield in influenza infection. In the appropriate clinical setting, pandemic strain-specific nucleic acid testing is the initial test of choice for suspected pandemic influenza. It is more sensitive than virus isolation, and more sensitive and specific than serology, immunofluorescence and other antigen detection methods. Virus isolation is needed to monitor new influenza strains and for vaccine development. Analysis of influenza isolates is undertaken by the World Health Organization Global Influenza Surveillance Network. Monitoring for antiviral resistance will be needed with widespread use of neuraminidase inhibitors for treatment and prophylaxis during a pandemic.
Figures
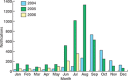

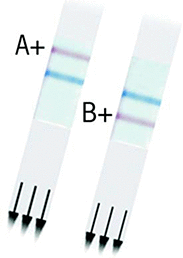
Similar articles
-
[Diagnostics of viral respiratory infections in hospitalized patients and ambulatory patients from SENTINEL program during 2004/05 season in Poland].Pol Arch Med Wewn. 2005 Oct;114(4):958-67. Pol Arch Med Wewn. 2005. PMID: 16789521 Polish.
-
Repeated seasonal influenza vaccination among elderly in Europe: Effects on laboratory confirmed hospitalised influenza.Vaccine. 2017 Aug 3;35(34):4298-4306. doi: 10.1016/j.vaccine.2017.06.088. Epub 2017 Jul 11. Vaccine. 2017. PMID: 28709555
-
Effectiveness of trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2012/13 end of season results.Euro Surveill. 2014 Jul 10;19(27):5-13. Euro Surveill. 2014. PMID: 25033051
-
[Epidemiological surveillance of influenza. Principles, means, possibilities].Bacteriol Virusol Parazitol Epidemiol. 2007 Jul-Dec;52(3-4):163-80. Bacteriol Virusol Parazitol Epidemiol. 2007. PMID: 19326730 Review. Romanian. No abstract available.
-
Fourth meeting of the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network and first scientific conference on acute respiratory infections in the Eastern Mediterranean Region, 11-14 December, 2017, Amman, Jordan.J Infect Public Health. 2020 Mar;13(3):451-456. doi: 10.1016/j.jiph.2020.02.031. Epub 2020 Mar 3. J Infect Public Health. 2020. PMID: 32144017 Free PMC article. Review.
Cited by
-
Pilot study of influenza vaccine effectiveness in urban Australian children attending childcare.J Paediatr Child Health. 2011 Dec;47(12):857-62. doi: 10.1111/j.1440-1754.2011.02098.x. Epub 2011 Jun 10. J Paediatr Child Health. 2011. PMID: 21658144 Free PMC article.
-
Evaluation of the Xpert Flu A Panel nucleic acid amplification-based point-of-care test for influenza A virus detection and pandemic H1 subtyping.J Clin Virol. 2010 Oct;49(2):85-9. doi: 10.1016/j.jcv.2010.07.005. Epub 2010 Jul 31. J Clin Virol. 2010. PMID: 20674478 Free PMC article.
-
Diagnostic testing or empirical therapy for patients hospitalized with suspected influenza: what to do?Clin Infect Dis. 2009 Jan 1;48 Suppl 1(Suppl 1):S14-9. doi: 10.1086/591852. Clin Infect Dis. 2009. PMID: 19067610 Free PMC article. Review.
-
Surveillance and clinical characterization of influenza in a university cohort in Singapore.PLoS One. 2015 Mar 19;10(3):e0119485. doi: 10.1371/journal.pone.0119485. eCollection 2015. PLoS One. 2015. PMID: 25790305 Free PMC article.
-
Buccal swabs as non-invasive specimens for detection of severe acute respiratory syndrome coronavirus-2.J Int Med Res. 2021 May;49(5):3000605211016996. doi: 10.1177/03000605211016996. J Int Med Res. 2021. PMID: 34027696 Free PMC article.
References
-
- Playford EG, Dwyer DE. Laboratory diagnosis of influenza virus infection. Pathology 2002; 34: 115–125. . - PubMed
-
- Nicholson KG. Human influenza In: Nicholson KG, Webster RG, Hays AJ, editors. Textbook of influenza. Oxford: Blackwell Science, 1998.
-
- World Health Organization Collaborating Centre for Reference and Research on Influenza, Melbourne [website]. https://www.influenzacentre.org (accessed Oct 2006).
-
- Donaldson A, Lewis FA, Kennett ML, et al. The 1976 influenza epidemic in Melbourne. Med J Aust 1978; 2: 45–49. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

