Increased proteolysis of diphtheria toxin by human monocytes after heat shock: a subsidiary role for heat-shock protein 70 in antigen processing
- PMID: 17116171
- PMCID: PMC2265859
- DOI: 10.1111/j.1365-2567.2006.02494.x
Increased proteolysis of diphtheria toxin by human monocytes after heat shock: a subsidiary role for heat-shock protein 70 in antigen processing
Abstract
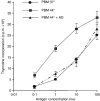
The expression of heat-shock proteins (hsp) increases after exposure to various stresses including elevated temperatures, oxidative injury, infection and inflammation. As molecular chaperones, hsp have been shown to participate in antigen processing and presentation, in part through increasing the stability and expression of major histocompatibility complex molecules. Heat shock selectively increases human T-cell responses to processed antigen, but does not affect T-cell proliferation induced by non-processed antigens. Here, we have analysed the mechanisms by which stress such as heat shock, and the ensuing hsp over-expression affect the processing of diphtheria toxin (DT) in peripheral blood monocytes. We found that heat shock increased DT proteolysis in endosomes and lysosomes while the activities of the cathepsins B and D, classically involved in DT proteolysis, were decreased. These effects correlated with the heat-shock-mediated increase in hsp 70 expression observed in endosomes and lysosomes. Actinomycin D or blocking anti-hsp 70 antibodies abolished the heat-shock-mediated increase in DT proteolysis. These data indicate that the increased expression of hsp 70 constitutes a subsidiary mechanism that facilitates antigen proteolysis in stressed cells. Confirming these data, presentation by formaldehyde-fixed cells of DT proteolysates that were obtained with endosomes and lysosomes from heat-shocked peripheral blood monocytes showed higher stimulation of T cells than those generated with endosomes and lysosomes from control peripheral blood monocytes.
Figures






References
-
- Rothman JE. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989;59:591–601. - PubMed
-
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355(6355):33–45. - PubMed
-
- Liu RY, Li X, Li L, Li GC. Expression of human hsp70 in rat fibroblasts enhances cell survival and facilitates recovery from translational and transcriptional inhibition following heat shock. Cancer Res. 1992;52:3667–73. - PubMed
-
- Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS. Protective effects of hsp70 in inflammation. Experientia. 1994;50:1031–8. - PubMed
-
- Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

