Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism
- PMID: 17116663
- PMCID: PMC1797738
- DOI: 10.1128/AAC.00853-06
Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism
Abstract
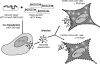
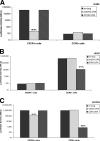
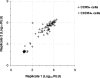
Most human immunodeficiency virus type 1 (HIV-1) strains require either the CXCR4 or CCR5 chemokine receptor to efficiently enter cells. Blocking viral binding to these coreceptors is an attractive therapeutic target. Currently, several coreceptor antagonists are being evaluated in clinical trials that require characterization of coreceptor tropism for enrollment. In this report, we describe the development of an automated and accurate procedure for determining HIV-1 coreceptor tropism (Trofile) and its validation for routine laboratory testing. HIV-1 pseudoviruses are generated using full-length env genes derived from patient virus populations. Coreceptor tropism is determined by measuring the abilities of these pseudovirus populations to efficiently infect CD4+/U87 cells expressing either the CXCR4 or CCR5 coreceptor. Viruses exclusively and efficiently infecting CXCR4+/CD4+/U87 cells are designated X4-tropic. Conversely, viruses exclusively and efficiently infecting CCR5+/CD4+/U87 cells are designated R5-tropic. Viruses capable of infecting both CXCR4+/CD4+/U87 and CCR5+/CD4+/U87 cells are designated dual/mixed-tropic. Assay accuracy and reproducibility were established by evaluating the tropisms of well-characterized viruses and the variability among replicate results from samples tested repeatedly. The viral subtype, hepatitis B virus or hepatitis C virus coinfection, and the plasma viral load did not affect assay performance. Minority subpopulations with alternate tropisms were reliably detected when present at 5 to 10%. The plasma viral load above which samples can be amplified efficiently in the Trofile assay is 1,000 copies per ml of plasma. Trofile has been automated for high-throughput use; it can be used to identify patients most likely to benefit from treatment regimens that include a coreceptor inhibitor and to monitor patients on treatment for the emergence of resistant virus populations that switch coreceptor tropism.
Figures
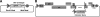
References
-
- Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091-1094. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Briz, V., E. Poveda, and V. Soriano. 2006. HIV entry inhibitors: mechanisms of action and resistance pathways. J. Antimicrob. Chemother. 57:619-627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

