High-throughput Plasmodium falciparum growth assay for malaria drug discovery
- PMID: 17116676
- PMCID: PMC1797774
- DOI: 10.1128/AAC.01144-06
High-throughput Plasmodium falciparum growth assay for malaria drug discovery
Abstract
New therapeutic agents for the treatment of malaria, the world's most deadly parasitic disease, are urgently needed. Malaria afflicts 300 to 500 million people and results in 1 to 2 million deaths annually, and more than 85% of all malaria-related mortality involves young children and pregnant women in sub-Saharan Africa. The emergence of multidrug-resistant parasites, especially in Plasmodium falciparum, has eroded the efficacy of almost all currently available therapeutic agents. The discovery of new drugs, including drugs with novel cellular targets, could be accelerated with a whole-organism high-throughput screen (HTS) of structurally diverse small-molecule libraries. The standard whole-organism screen is based on incorporation of [3H]hypoxanthine and has liabilities, such as limited throughput, high cost, multiple labor-intensive steps, and disposal of radioactive waste. Recently, screens have been reported that do not use radioactive incorporation, but their reporter signal is not robust enough for HTS. We report a P. falciparum growth assay that is technically simple, robust, and compatible with the automation necessary for HTS. The assay monitors DNA content by addition of the fluorescent dye 4',6-diamidino-2-phenylindole (DAPI) as a reporter of blood-stage parasite growth. This DAPI P. falciparum growth assay was used to measure the 50% inhibitory concentrations (IC50s) of a diverse set of known antimalarials. The resultant IC50s compared favorably with those obtained in the [3H]hypoxanthine incorporation assay. Over 79,000 small molecules have been tested for antiplasmodial activity using the DAPI P. falciparum growth assay, and 181 small molecules were identified as highly active against multidrug-resistant parasites.
Figures
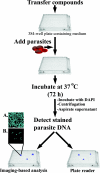

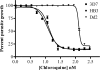
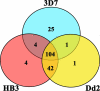
References
-
- Bennett, T. N., A. D. Kosar, L. M. Ursos, S. Dzekunov, A. B. Singh Sidhu, D. A. Fidock, and P. D. Roepe. 2004. Drug resistance-associated pfCRT mutations confer decreased Plasmodium falciparum digestive vacuolar pH. Mol. Biochem. Parasitol. 133:99-114. - PubMed
-
- Blackwell, H. E., L. Perez, and S. L. Schreiber. 2001. Decoding products of diversity pathways from stock solutions derived from single polymeric macrobeads. Angew. Chem. Int. Ed. Engl. 40:3421-3425. - PubMed
-
- Blackwell, H. E., L. Perez, R. A. Stavenger, J. A. Tallarico, E. Cope Eatough, M. A. Foley, and S. L. Schreiber. 2001. A one-bead, one-stock solution approach to chemical genetics: part 1. Chem. Biol. 8:1167-1182. - PubMed
-
- Clark, A. G. 2002. Population genetics: malaria variorum. Nature 418:283-285. - PubMed
-
- Corbett, Y., L. Herrera, J. Gonzalez, L. Cubilla, T. L. Capson, P. D. Coley, T. A. Kursar, L. I. Romero, and E. Ortega-Barria. 2004. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am. J. Trop. Med. Hyg. 70:119-124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

