Intramolecular regulation of phospholipase C-gamma1 by its C-terminal Src homology 2 domain
- PMID: 17116690
- PMCID: PMC1800685
- DOI: 10.1128/MCB.01400-06
Intramolecular regulation of phospholipase C-gamma1 by its C-terminal Src homology 2 domain
Abstract
Phosphoinositide-specific phospholipase C-gamma1 (PLC-gamma1) is a key enzyme that governs cellular functions such as gene transcription, secretion, proliferation, motility, and development. Here, we show that PLC-gamma1 is regulated via a novel autoinhibitory mechanism involving its carboxy-terminal Src homology (SH2C) domain. Mutation of the SH2C domain tyrosine binding site led to constitutive PLC-gamma1 activation. The amino-terminal split pleckstrin homology (sPHN) domain was found to regulate the accessibility of the SH2C domain. PLC-gamma1 constructs with mutations in tyrosine 509 and phenylalanine 510 in the sPHN domain no longer required an intact amino-terminal Src homology (SH2N) domain or phosphorylation of tyrosine 775 or 783 for activation. These data are consistent with a model in which the SH2C domain is blocked by an intramolecular interaction(s) that is released upon cellular activation by occupancy of the SH2N domain.
Figures

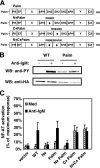
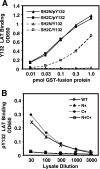
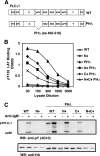
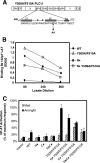
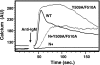
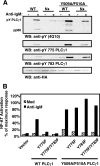

 ). Once released from intramolecular inhibition, the catalytic domains act in concert to hydrolyze PI(4,5)P2 (PIP2).
). Once released from intramolecular inhibition, the catalytic domains act in concert to hydrolyze PI(4,5)P2 (PIP2).References
-
- Carpenter, G., and Q. Ji. 1999. Phospholipase C-gamma as a signal-transducing element. Exp. Cell Res. 253:15-24. - PubMed
-
- Chang, J. S., H. Seok, T. K. Kwon, D. S. Min, B. H. Ahn, Y. H. Lee, J. W. Suh, J. W. Kim, S. Iwashita, A. Omori, S. Ichinose, O. Numata, J. K. Seo, Y. S. Oh, and P. G. Suh. 2002. Interaction of elongation factor-1alpha and pleckstrin homology domain of phospholipase C-gamma 1 with activating its activity. J. Biol. Chem. 277:19697-19702. - PubMed
-
- Choi, J. H., W. P. Hong, S. Yun, H. S. Kim, J. R. Lee, J. B. Park, Y. S. Bae, S. H. Ryu, and P. G. Suh. 2005. Grb2 negatively regulates epidermal growth factor-induced phospholipase C-gamma1 activity through the direct interaction with tyrosine-phosphorylated phospholipase C-gamma1. Cell. Signal. 17:1289-1299. - PubMed
-
- Crabtree, G. R., and N. A. Clipstone. 1994. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu. Rev. Biochem. 63:1045-1083. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
