Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men
- PMID: 17116734
- PMCID: PMC2118165
- DOI: 10.1084/jem.20060667
Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men
Abstract
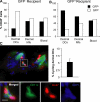
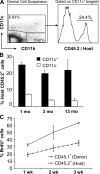
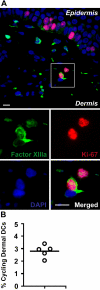
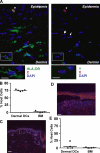
In this study, we explored dermal dendritic cell (DC) homeostasis in mice and humans both in the steady state and after hematopoietic cell transplantation. We discovered that dermal DCs proliferate in situ in mice and human quiescent dermis. In parabiotic mice with separate organs but shared blood circulation, the majority of dermal DCs failed to be replaced by circulating precursors for >6 mo. In lethally irradiated mice injected with donor congenic bone marrow (BM) cells, a subset of recipient DCs remained in the dermis and proliferated locally throughout life. Consistent with these findings, a large proportion of recipient dermal DCs remained in patients' skin after allogeneic hematopoietic cell transplantation, despite complete donor BM chimerism. Collectively, our results oppose the traditional view that DCs are nondividing terminally differentiated cells maintained by circulating precursors and support the new paradigm that tissue DCs have local proliferative properties that control their homeostasis in the steady state. Given the role of residual host tissue DCs in transplant immune reactions, these results suggest that dermal DC homeostasis may contribute to the development of cutaneous graft-versus-host disease in clinical transplantation.
Figures



References
-
- Valladeau, J., and S. Saeland. 2005. Cutaneous dendritic cells. Semin. Immunol. 17:273–283. - PubMed
-
- Schuler, G., F. Koch, C. Heufler, E. Kampgen, G. Topar, and N. Romani. 1993. Murine epidermal Langerhans cells as a model to study tissue dendritic cells. Adv. Exp. Med. Biol. 329:243–249. - PubMed
-
- Cerio, R., C.E. Griffiths, K.D. Cooper, B.J. Nickoloff, and J.T. Headington. 1989. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br. J. Dermatol. 121:421–431. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

