Random migration precedes stable target cell interactions of tumor-infiltrating T cells
- PMID: 17116735
- PMCID: PMC2118164
- DOI: 10.1084/jem.20060710
Random migration precedes stable target cell interactions of tumor-infiltrating T cells
Abstract
The tumor microenvironment is composed of an intricate mixture of tumor and host-derived cells that engage in a continuous interplay. T cells are particularly important in this context as they may recognize tumor-associated antigens and induce tumor regression. However, the precise identity of cells targeted by tumor-infiltrating T lymphocytes (TILs) as well as the kinetics and anatomy of TIL-target cell interactions within tumors are incompletely understood. Furthermore, the spatiotemporal conditions of TIL locomotion through the tumor stroma, as a prerequisite for establishing contact with target cells, have not been analyzed. These shortcomings limit the rational design of immunotherapeutic strategies that aim to overcome tumor-immune evasion. We have used two-photon microscopy to determine, in a dynamic manner, the requirements leading to tumor regression by TILs. Key observations were that TILs migrated randomly throughout the tumor microenvironment and that, in the absence of cognate antigen, they were incapable of sustaining active migration. Furthermore, TILs in regressing tumors formed long-lasting (>or=30 min), cognate antigen-dependent contacts with tumor cells. Finally, TILs physically interacted with macrophages, suggesting tumor antigen cross-presentation by these cells. Our results demonstrate that recognition of cognate antigen within tumors is a critical determinant of optimal TIL migration and target cell interactions, and argue against TIL guidance by long-range chemokine gradients.
Figures
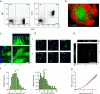

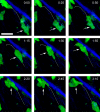
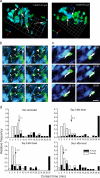
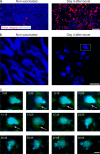
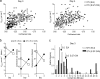
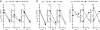
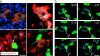
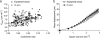
References
-
- Dunn, G.P., L.J. Old, and R.D. Schreiber. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329–360. - PubMed
-
- Jager, E., D. Jager, and A. Knuth. 2003. Antigen-specific immunotherapy and cancer vaccines. Int. J. Cancer. 106:817–820. - PubMed
-
- Ho, W.Y., J.N. Blattman, M.L. Dossett, C. Yee, and P.D. Greenberg. 2003. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 3:431–437. - PubMed
-
- Sadelain, M., I. Riviere, and R. Brentjens. 2003. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 3:35–45. - PubMed
-
- Schuler, G., B. Schuler-Thurner, and R.M. Steinman. 2003. The use of dendritic cells in cancer immunotherapy. Curr. Opin. Immunol. 15:138–147. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

