Spontaneous autoimmunity prevented by thymic expression of a single self-antigen
- PMID: 17116738
- PMCID: PMC2118158
- DOI: 10.1084/jem.20061864
Spontaneous autoimmunity prevented by thymic expression of a single self-antigen
Erratum in
- J Exp Med. 2007 Jan 22;204(1):203. Caspi, Rachel [corrected to Caspi, Rachel R]
Abstract
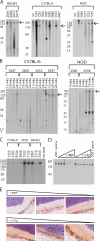
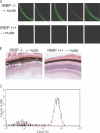
The expression of self-antigen in the thymus is believed to be responsible for the deletion of autoreactive T lymphocytes, a critical process in the maintenance of unresponsiveness to self. The Autoimmune regulator (Aire) gene, which is defective in the disorder autoimmune polyglandular syndrome type 1, has been shown to promote the thymic expression of self-antigens. A clear link, however, between specific thymic self-antigens and a single autoimmune phenotype in this model has been lacking. We show that autoimmune eye disease in aire-deficient mice develops as a result of loss of thymic expression of a single eye antigen, interphotoreceptor retinoid-binding protein (IRBP). In addition, lack of IRBP expression solely in the thymus, even in the presence of aire expression, is sufficient to trigger spontaneous eye-specific autoimmunity. These results suggest that failure of thymic expression of selective single self-antigens can be sufficient to cause organ-specific autoimmune disease, even in otherwise self-tolerant individuals.
Figures

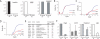

References
-
- Kappler, J.W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell. 49:273–280. - PubMed
-
- Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. - PubMed
-
- Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. - PubMed
-
- Liston, A., S. Lesage, J. Wilson, L. Peltonen, and C.C. Goodnow. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350–354. - PubMed
-
- Nagamine, K., P. Peterson, H.S. Scott, J. Kudoh, S. Minoshima, M. Heino, K.J. Krohn, M.D. Lalioti, P.E. Mullis, S.E. Antonarakis, et al. 1997. Positional cloning of the APECED gene. Nat. Genet. 17:393–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

