Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function
- PMID: 17116885
- PMCID: PMC1654129
- DOI: 10.1073/pnas.0609251103
Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function
Abstract
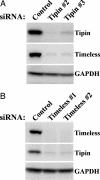
Tipin is a mammalian protein that interacts with Timeless, which plays a role in DNA damage checkpoint responses. Here, we show that Tipin is a nuclear protein that associates with the replicative helicase and protects cells against genotoxic agents. Tipin is required for efficient cell cycle arrest in response to DNA damage, and depletion of Tipin renders cells sensitive to ionizing radiation as well as replication stress. Loss of Tipin results in spontaneous gamma-H2AX foci, a marker for DNA double-strand breaks. We find that Tipin and Timeless form a complex that maintains the level of both proteins in cells and that the loss of either one will lead to the loss of the interacting partner. This observation explains the similar checkpoint phenotypes observed in both Tipin- and Timeless-depleted cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hardin PE. Curr Biol. 2005;15:R714–R722. - PubMed
-
- Koike N, Hida A, Numano R, Hirose M, Sakaki Y, Tei H. FEBS Lett. 1998;441:427–431. - PubMed
-
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, et al. Neuron. 1998;21:1101–1113. - PubMed
-
- Chan RC, Chan A, Jeon M, Wu TF, Pasqualone D, Rougvie AE, Meyer BJ. Nature. 2003;423:1002–1009. - PubMed
-
- Benna C, Scannapieco P, Piccin A, Sandrelli F, Zordan M, Rosato E, Kyriacou CP, Valle G, Costa R. Curr Biol. 2000;10:R512–R513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

