Costs and benefits of processivity in enzymatic degradation of recalcitrant polysaccharides
- PMID: 17116887
- PMCID: PMC1838711
- DOI: 10.1073/pnas.0608909103
Costs and benefits of processivity in enzymatic degradation of recalcitrant polysaccharides
Abstract
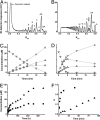
Many enzymes that hydrolyze insoluble crystalline polysaccharides such as cellulose and chitin guide detached single-polymer chains through long and deep active-site clefts, leading to processive (stepwise) degradation of the polysaccharide. We have studied the links between enzyme efficiency and processivity by analyzing the effects of mutating aromatic residues in the substrate-binding groove of a processive chitobiohydrolase, chitinase B from Serratia marcescens. Mutation of two tryptophan residues (Trp-97 and Trp-220) close to the catalytic center (subsites +1 and +2) led to reduced processivity and a reduced ability to degrade crystalline chitin, suggesting that these two properties are linked. Most remarkably, the loss of processivity in the W97A mutant was accompanied by a 29-fold increase in the degradation rate for single-polymer chains as present in the soluble chitin-derivative chitosan. The properties of the W220A mutant showed a similar trend, although mutational effects were less dramatic. Processivity is thought to contribute to the degradation of crystalline polysaccharides because detached single-polymer chains are kept from reassociating with the solid material. The present results show that this processivity comes at a large cost in terms of enzyme speed. Thus, in some cases, it might be better to focus strategies for enzymatic depolymerization of polysaccharide biomass on improving substrate accessibility for nonprocessive enzymes rather than on improving the properties of processive enzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Aromatic residues in the catalytic center of chitinase A from Serratia marcescens affect processivity, enzyme activity, and biomass converting efficiency.J Biol Chem. 2009 Apr 17;284(16):10610-7. doi: 10.1074/jbc.M900092200. Epub 2009 Feb 25. J Biol Chem. 2009. PMID: 19244232 Free PMC article.
-
Treatment of recalcitrant crystalline polysaccharides with lytic polysaccharide monooxygenase relieves the need for glycoside hydrolase processivity.Carbohydr Res. 2019 Feb 1;473:66-71. doi: 10.1016/j.carres.2019.01.001. Epub 2019 Jan 7. Carbohydr Res. 2019. PMID: 30640029
-
Enzyme processivity changes with the extent of recalcitrant polysaccharide degradation.FEBS Lett. 2014 Dec 20;588(24):4620-4. doi: 10.1016/j.febslet.2014.10.034. Epub 2014 Nov 5. FEBS Lett. 2014. PMID: 25447535
-
The chitinolytic machinery of Serratia marcescens--a model system for enzymatic degradation of recalcitrant polysaccharides.FEBS J. 2013 Jul;280(13):3028-49. doi: 10.1111/febs.12181. Epub 2013 Mar 7. FEBS J. 2013. PMID: 23398882 Review.
-
Bacterial Chitinase System as a Model of Chitin Biodegradation.Adv Exp Med Biol. 2019;1142:131-151. doi: 10.1007/978-981-13-7318-3_7. Adv Exp Med Biol. 2019. PMID: 31102245 Review.
Cited by
-
Modeling the activity burst in the initial phase of cellulose hydrolysis by the processive cellobiohydrolase Cel7A.Biotechnol Bioeng. 2019 Mar;116(3):515-525. doi: 10.1002/bit.26889. Epub 2019 Jan 8. Biotechnol Bioeng. 2019. PMID: 30515756 Free PMC article.
-
Proteolytic release of the intramolecular chaperone domain confers processivity to endosialidase F.J Biol Chem. 2009 Apr 3;284(14):9465-74. doi: 10.1074/jbc.M808475200. Epub 2009 Feb 3. J Biol Chem. 2009. PMID: 19189967 Free PMC article.
-
Cellobiohydrolase and endoglucanase respond differently to surfactants during the hydrolysis of cellulose.Biotechnol Biofuels. 2015 Mar 28;8:52. doi: 10.1186/s13068-015-0242-y. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 25829946 Free PMC article.
-
Chitosans for delivery of nucleic acids.Adv Drug Deliv Rev. 2013 Aug;65(9):1234-70. doi: 10.1016/j.addr.2013.07.005. Epub 2013 Jul 18. Adv Drug Deliv Rev. 2013. PMID: 23872012 Free PMC article. Review.
-
A novel thermostable chitinolytic machinery of Streptomyces sp. F-3 consisting of chitinases with different action modes.Biotechnol Biofuels. 2019 Jun 3;12:136. doi: 10.1186/s13068-019-1472-1. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31171937 Free PMC article.
References
-
- Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, et al. Science. 2006;311:484–489. - PubMed
-
- Farrell AE, Plevin RJ, Turner BT, Jones AD, O'Hare M, Kammen DM. Science. 2006;311:5006–5008. - PubMed
-
- Peter MG. In: Biopolymers, Vol. 6: Polysaccharides II. De Baets S, Vandamme EJ, Steinbüchel A, editors. Weinheim, Germany: Wiley; 2002. pp. 481–574.
-
- Langer RC, Vinetz JM. Trends Parasitol. 2001;17:269–272. - PubMed
-
- Hawtin RE, Zarkowska T, Arnold K, Thomas CJ, Gooday GW, King LA, Kuzio JA, Possee RD. Virology. 1997;238:243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

