Chemical coding of myenteric neurons with different axonal projection patterns in the porcine ileum
- PMID: 17118061
- PMCID: PMC2049006
- DOI: 10.1111/j.1469-7580.2006.00653.x
Chemical coding of myenteric neurons with different axonal projection patterns in the porcine ileum
Abstract
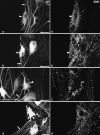
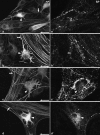
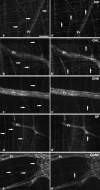
The aim of this study was to perform an immunohistochemical characterization of two different myenteric neuron types of the pig displaying opposite axonal projections. These were type I neurons equipped with lamellar dendrites that projected mainly orally, and type VI neurons that displayed typical axonal dendrites and projected anally. Double immunostainings of longitudinal muscle/myenteric plexus wholemounts from ileal segments of four pigs were performed to visualize neurofilaments (NF) in combination with calcitonin gene-related peptide (CGRP), leu-enkephalin (ENK) and substance P (SP), respectively. Triple immunostainings of wholemounts, using antibodies against neuronal nitric oxide synthase (nNOS) and vasoactive intestinal peptide (VIP) as well as against VIP and galanin (GAL), were performed. We found that 78% of type I neurons immunoreacted to ENK, 21% to CGRP and 24% to SP. The NF-positive type I neurons co-reactive for one of the three above markers displayed mostly frayed outlines of both their somal contours and their broadened dendritic endings. By contrast, most of the non-coreactive type I neurons displayed rather sharply outlined somata and dendrites. No type I neuron immunoreacted to nNOS, VIP or GAL and none of the type VI NF-reactive neurons reacted to CGRP, ENK or SP. All type VI neurons investigated displayed immunoreactivity for nNOS, 92% of which were co-reactive for VIP. Co-reactivity for VIP and GAL was found in 69% of type VI neurons, 21% were positive for VIP but negative for GAL, 9% were negative for both GAL and VIP, and 1% were positive for GAL but negative for VIP. We conclude that there are two subpopulations of morphological type I neurons. One of these displays mainly oral projections and could not be further characterized in this study. The other, which may correspond to neurons innervating the longitudinal and circular muscle layers, were partly immunoreactive for ENK, CGRP and/or SP. Type VI neurons are immunoreactive for nNOS frequently co-localized with VIP and, partly, also GAL. These may be inhibitory motor neurons and are different from VIP/GAL-coreactive minineurons described earlier.
Figures


Similar articles
-
Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut.Histochem Cell Biol. 2006 May;125(5):557-65. doi: 10.1007/s00418-005-0107-8. Epub 2005 Nov 19. Histochem Cell Biol. 2006. PMID: 16328433
-
Chemical coding of submucosal type V neurons in porcine ileum.Cells Tissues Organs. 2006;184(1):31-41. doi: 10.1159/000096949. Cells Tissues Organs. 2006. PMID: 17190978
-
The longitudinal smooth muscle layer of the pig small intestine is innervated by both myenteric and submucous neurons.Histochem Cell Biol. 2002 Jun;117(6):481-92. doi: 10.1007/s00418-002-0406-2. Epub 2002 Apr 27. Histochem Cell Biol. 2002. PMID: 12107499
-
Projections of enteric peptide-containing neurons in the rat.Arch Histol Cytol. 1989;52 Suppl:181-9. doi: 10.1679/aohc.52.suppl_181. Arch Histol Cytol. 1989. PMID: 2479401 Review.
-
Structural and chemical organization of the myenteric plexus.Annu Rev Physiol. 1988;50:81-93. doi: 10.1146/annurev.ph.50.030188.000501. Annu Rev Physiol. 1988. PMID: 3288110 Review.
Cited by
-
Age and Sex-Dependent Differences in the Neurochemical Characterization of Calcitonin Gene-Related Peptide-Like Immunoreactive (CGRP-LI) Nervous Structures in the Porcine Descending Colon.Int J Mol Sci. 2019 Feb 27;20(5):1024. doi: 10.3390/ijms20051024. Int J Mol Sci. 2019. PMID: 30818742 Free PMC article.
-
Endogenous opiates and behavior: 2006.Peptides. 2007 Dec;28(12):2435-513. doi: 10.1016/j.peptides.2007.09.002. Epub 2007 Sep 11. Peptides. 2007. PMID: 17949854 Free PMC article. Review.
-
Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio).J Comp Neurol. 2010 Nov 1;518(21):4419-38. doi: 10.1002/cne.22464. J Comp Neurol. 2010. PMID: 20853514 Free PMC article.
References
-
- Brehmer A, Stach W. Morphological classification of NADPHd-positive and – negative myenteric neurons in the porcine small intestine. Cell Tissue Res. 1997;287:127–134. - PubMed
-
- Brehmer A, Stach W, Krammer H-J, Neuhuber W. Distribution, morphology and projections of nitrergic and non-nitrergic submucosal neurons in the pig small intestine. Histochem Cell Biol. 1998;109:87–94. - PubMed
-
- Brehmer A, Schrödl F, Neuhuber W. Morphological classifications of enteric neurons – 100 years after Dogiel. Anat Embryol. 1999;200:125–135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

