Cellular and molecular regulation of the primate endometrium: a perspective
- PMID: 17118167
- PMCID: PMC1775063
- DOI: 10.1186/1477-7827-4-S1-S3
Cellular and molecular regulation of the primate endometrium: a perspective
Abstract
This contribution will trace some of the many seminal studies on the female uterus (endometrium) over the centuries and conclude with a description of some current research initiatives in our laboratory. Numerous contributions from many investigators over the years have contributed to our current understanding of endometrial function. The historical section of this chapter is intended to be a brief overall description of some of these efforts and not exhaustive. Additional information can be found in the review articles and books cited herein.
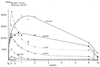
Figures















References
-
- Ramsey EM. History. In: Wynn RM, editor. Biology of the Uterus. New York and London: Plenum Press; 1977. pp. 1–18.
-
- Medvei CM. In: A History of Endocrinology. Hingham MA, editor. MTP Press; 1982.
-
- Young WC. Edgar Allen. In: Young WC, Corner GW, editor. Sex and Internal Secretions. Baltimore: Williams & Wilkins; 1961. pp. xiii–xix.
-
- Allen E, Doisy EA. The extraction and some properties of an ovarian hormone. J Biol Chem. 1924;61:7–27.
-
- Allen E, Doisy EA. The induction of a sexually mature condition in immature females by injection of the ovarian follicular hormone. Am J Physiol. 1924;69:577–588.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

