A critical period of progesterone withdrawal precedes menstruation in macaques
- PMID: 17118170
- PMCID: PMC1775066
- DOI: 10.1186/1477-7827-4-S1-S6
A critical period of progesterone withdrawal precedes menstruation in macaques
Abstract
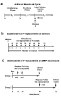
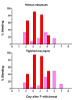
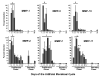
Macaques are menstruating nonhuman primates that provide important animal models for studies of hormonal regulation in the uterus. In women and macaques the decline of progesterone (P) at the end of the cycle triggers endometrial expression of a variety of matrix metalloproteinase (MMP) enzymes that participate in tissue breakdown and menstrual sloughing. To determine the minimal duration of P withdrawal required to induce menses, we assessed the effects of adding P back at various time points after P withdrawal on both frank bleeding patterns and endometrial MMP expression. Artificial menstrual cycles were induced by treating the animals sequentially with implants releasing estradiol (E2) and progesterone (P). To assess bleeding patterns, P implants were removed at the end of a cycle and then added back at 12, 24, 30, 36, 40, 48, 60, or 72 hours (h) after the initial P withdrawal. Observational analysis of frank bleeding patterns showed that P replacement at 12 and 24 h blocked menses, replacement at 36 h reduced menses but replacement after 36 h failed to block menses. These data indicate that in macaques, a critical period of P withdrawal exists and lasts approximately 36 h. In other similarly cycled animals, we withdrew P and then added P back either during (12-24 h) or after (48 h) the critical period, removed the uterus 24 h after P add back and evaluated endometrial MMP expression. Immunocytochemistry showed that replacement of P during the critical period suppressed MMP-1, -2 and -3 expression along with menses, but replacement of P at 48 h, which failed to suppress mense, suppressed MMP-1 and MMP-3 but did not block MMP-2. We concluded that upregulation of MMPs is essential to menses induction, but that after the critical period, menses will occur even if some MMPs are experimentally blocked.
Figures






References
-
- Brenner RM, McClellan MC, West NB, Novy MJ, Haluska GJ, Sternfeld MD. Estrogen and progestin receptors in the macaque endometrium. In: Bulletti C, Gurpide E, editor. The Primate Endometrium. NY: New York Academy of Sciences; 1991. pp. 149–166. - PubMed
-
- Brenner RM, Slayden OD. Cyclic changes in the primate oviduct and endometrium. In: Knobil E, Neill JD, editor. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 541–569.
-
- Bartelmez GW. Cyclic changes in the endometrium of the rhesus monkey (Macaca mulatta) Contrib Embryol. 1951;34:99–144.
-
- Bartelmez GW. The phases of the menstrual cycle and their interpretation in terms of the pregnancy cycle. Am J Obstet Gynecol. 1957;74:931–955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

