Increased expression and activity of repair genes TDP1 and XPF in non-small cell lung cancer
- PMID: 17118488
- PMCID: PMC1890013
- DOI: 10.1016/j.lungcan.2006.10.019
Increased expression and activity of repair genes TDP1 and XPF in non-small cell lung cancer
Abstract
Resistance to camptothecin (CPT), a topoisomerase I (Top1) inhibitor, is frequently encountered in non-small cell lung cancer (NSCLC) and CPT resistance is linked with TDP1, an enzyme capable of cleaving the covalent linkage between stabilized Top1 with DNA. The aim of this study is to evaluate the in vivo expression level of TDP1, as well as parallel repair pathway components XPF and MUS81, in primary NSCLC. We collected 30 un-matched and 4 NSCLC samples matched with normal lung tissue and 8 samples of non-neoplastic lung tissue from patients with and without lung cancer, and determined the protein expression of these three genes using Western blot and TDP1 activity by a specific enzymatic assay. Both TDP1 and XPF were overexpressed in over 50% of NSCLC tissues, with wide ranges of expression levels. MUS81 did not exhibit alteration in expression. Overexpression of TDP1 and XPF is common in NSCLC, and is therefore of interest as a possible contributor to drug resistance in NSCLC.
Conflict of interest statement
Conflict of interest statement
None declared
Figures
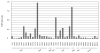









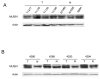
References
-
- Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, et al. Class iii beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. - PubMed
-
- Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after pka activation in breast cancer. Cancer Cell. 2004;5:597–605. - PubMed
-
- Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ercc1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–91. - PubMed
-
- Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003;361:2235–42. - PubMed
-
- Stewart DJ. Update on the role of topotecan in the treatment of non-small cell lung cancer. Oncologist. 2004;9 (Suppl 6):43–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

