Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae
- PMID: 17118977
- PMCID: PMC1828485
- DOI: 10.1128/IAI.01604-06
Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae
Abstract
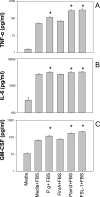
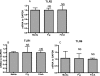
Humans develop periodontitis in response to challenge by microbial dental plaque. Inflammation begins after perturbation of gingival epithelial cells by subgingival bacteria interacting through pattern-recognition receptors, including the Toll-like receptors (TLR). Porphyromonas gingivalis is a major periodontopathogen that interacts with epithelial cells through its cell surface fimbriae (FimA), leading to colonization and/or invasion. Previous work by our group has established membrane CD14 as an essential coreceptor for TLR2-mediated activation of transfected cell lines by P. gingivalis FimA. We have shown that gingival epithelial cells express TLR2 but not CD14 on their cell surfaces. We thus speculated that P. gingivalis FimA does not readily activate epithelial innate immune responses but rather functions to promote P. gingivalis colonization in the absence of a vigorous FimA-induced response. This hypothesis was verified by the findings that primary human gingival epithelial cells responded poorly to FimA in terms of interleukin (IL)-6, IL-8, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha responses, in stark contrast to the marked response to other TLR2 agonists (Pam3Cys, FSL-1) that are not strictly dependent on CD14. On the other hand, CD14-expressing human primary monocytes responded with high levels of the same cytokines to both FimA and the control TLR2 agonists. The gingival epithelial cells failed to respond to FimA even in the presence of exogenously added soluble CD14. These data indicate that the gingival epithelial cell hyporesponsiveness to FimA is attributable to the lack of membrane-expressed but not soluble CD14. In conclusion, P. gingivalis FimA differentially activates human monocytes and epithelial cells, perhaps reflecting different tactics used by P. gingivalis when interacting with different host cell types or a host strategy to limit inflammation.
Figures




Similar articles
-
Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus.Cell Microbiol. 2006 Oct;8(10):1557-70. doi: 10.1111/j.1462-5822.2006.00730.x. Cell Microbiol. 2006. PMID: 16984411
-
Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis.J Periodontal Res. 2011 Aug;46(4):407-16. doi: 10.1111/j.1600-0765.2011.01353.x. Epub 2011 Feb 17. J Periodontal Res. 2011. PMID: 21332474
-
Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2.Infect Immun. 2001 Dec;69(12):7387-95. doi: 10.1128/IAI.69.12.7387-7395.2001. Infect Immun. 2001. PMID: 11705912 Free PMC article.
-
Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):90-6. doi: 10.1902/jop.2003.74.1.90. J Periodontol. 2003. PMID: 12593602 Review.
-
Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis.Ann Periodontol. 2002 Dec;7(1):72-8. doi: 10.1902/annals.2002.7.1.72. Ann Periodontol. 2002. PMID: 16013219 Review.
Cited by
-
MicroRNAs and Periodontal Disease: Helpful Therapeutic Targets?Adv Pharm Bull. 2023 Jul;13(3):423-434. doi: 10.34172/apb.2023.048. Epub 2022 Jul 2. Adv Pharm Bull. 2023. PMID: 37646047 Free PMC article. Review.
-
Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae.J Immunol. 2009 Jun 1;182(11):6690-6. doi: 10.4049/jimmunol.0900524. J Immunol. 2009. PMID: 19454663 Free PMC article.
-
A Periodontal pathogen Porphyromonas gingivalis deteriorates Isoproterenol-Induced myocardial remodeling in mice.Hypertens Res. 2017 Jan;40(1):35-40. doi: 10.1038/hr.2016.114. Epub 2016 Sep 8. Hypertens Res. 2017. PMID: 27604343
-
Sphingosine kinase-1 is required for toll mediated beta-defensin 2 induction in human oral keratinocytes.PLoS One. 2010 Jul 9;5(7):e11512. doi: 10.1371/journal.pone.0011512. PLoS One. 2010. PMID: 20634980 Free PMC article.
-
Complement-Dependent Mechanisms and Interventions in Periodontal Disease.Front Immunol. 2019 Mar 12;10:406. doi: 10.3389/fimmu.2019.00406. eCollection 2019. Front Immunol. 2019. PMID: 30915073 Free PMC article. Review.
References
-
- Andrian, E., D. Grenier, and M. Rouabhia. 2006. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J. Dent. Res. 85:392-403. - PubMed
-
- Guillot, L., S. Medjane, K. Le-Barillec, V. Balloy, C. Danel, M. Chignard, and M. Si-Tahar. 2004. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 279:2712-2718. - PubMed
-
- Hajishengallis, G., P. Ratti, and E. Harokopakis. 2005. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J. Biol. Chem. 280:38902-38913. - PubMed
-
- Hajishengallis, G., H. Sojar, R. J. Genco, and E. DeNardin. 2004. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immun. Investig. 33:157-172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

