Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages
- PMID: 17118979
- PMCID: PMC1828506
- DOI: 10.1128/IAI.01199-06
Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages
Abstract
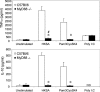
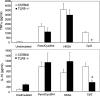
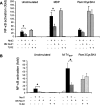
Toll-like receptors (TLRs) are involved in the sensing of microbially derived compounds. We analyzed the contribution of these receptors to cytokine production by macrophages following stimulation with whole bacteria. Using knockout mice, we confirmed that the TLR4 and TLR2 contribution was predominant in the induction of tumor necrosis factor alpha and interleukin-10 by gram-negative bacteria. In contrast, the absence of TLR2 and/or TLR4 or TLR9 did not affect the response to gram-positive bacteria. In the absence of TLR2, phagocytosis was essential for cytokine production in response to heat-killed Staphylococcus aureus (HKSA). Because intracellular sensing was important in the absence of TLR2, we evaluated the contribution of Nod1 and Nod2, intracytoplasmic sensors of peptidoglycan-derived muropeptides, to the response to HKSA. By transfecting RAW 264.7 macrophages with dominant negative (DN) forms of Nod1 and Nod2, we showed that both molecules inhibited NF-kappaB activation in response to HKSA. The unexpected interference of DN Nod1 in the response of macrophages to gram-positive bacteria was confirmed with a Nod2 agonist (muramyl dipeptide) in transfection experiments with HEK293T cell. Taken together, these results show the contribution of phagocytosis and Nod molecules to the response to HKSA in macrophages and also identify possible cross talk between Nod1 and Nod2.
Figures



Similar articles
-
The role of nucleotide-binding oligomerization domain 1 during cytokine production by macrophages in response to Mycobacterium tuberculosis infection.Immunobiology. 2016 Jan;221(1):70-5. doi: 10.1016/j.imbio.2015.07.020. Epub 2015 Jul 26. Immunobiology. 2016. PMID: 26255090
-
Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients.Crit Care Med. 2006 Sep;34(9):2377-85. doi: 10.1097/01.CCM.0000233875.93866.88. Crit Care Med. 2006. PMID: 16850005
-
Up-regulation of NOD1 and NOD2 through TLR4 and TNF-alpha in LPS-treated murine macrophages.J Vet Med Sci. 2006 May;68(5):471-8. doi: 10.1292/jvms.68.471. J Vet Med Sci. 2006. PMID: 16757890
-
Innate immune sensing of microbes by Nod proteins.Ann N Y Acad Sci. 2006 Aug;1072:19-27. doi: 10.1196/annals.1326.020. Ann N Y Acad Sci. 2006. PMID: 17057187 Review.
-
Intracellular NOD-like receptors in host defense and disease.Immunity. 2007 Oct;27(4):549-59. doi: 10.1016/j.immuni.2007.10.002. Immunity. 2007. PMID: 17967410 Review.
Cited by
-
Intracellular Staphylococcus aureus: live-in and let die.Front Cell Infect Microbiol. 2012 Apr 24;2:43. doi: 10.3389/fcimb.2012.00043. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919634 Free PMC article. Review.
-
Rethinking Communication in the Immune System: The Quorum Sensing Concept.Trends Immunol. 2019 Feb;40(2):88-97. doi: 10.1016/j.it.2018.12.002. Epub 2019 Jan 2. Trends Immunol. 2019. PMID: 30611647 Free PMC article. Review.
-
The R753Q polymorphism in Toll-like receptor 2 (TLR2) attenuates innate immune responses to mycobacteria and impairs MyD88 adapter recruitment to TLR2.J Biol Chem. 2017 Jun 23;292(25):10685-10695. doi: 10.1074/jbc.M117.784470. Epub 2017 Apr 25. J Biol Chem. 2017. PMID: 28442574 Free PMC article.
-
Cell-autonomous sex differences in gene expression in chicken bone marrow-derived macrophages.J Immunol. 2015 Mar 1;194(5):2338-44. doi: 10.4049/jimmunol.1401982. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637020 Free PMC article.
-
Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9.J Immunol. 2012 Oct 15;189(8):4040-6. doi: 10.4049/jimmunol.1201055. Epub 2012 Sep 7. J Immunol. 2012. PMID: 22962685 Free PMC article.
References
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Barnich, N., T. Hisamatsu, J. Aguirre, R. Xavier, H. Reinecker, and D. Podolsky. 2005. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J. Biol. Chem. 280:19021-19026. - PubMed
-
- Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014-1018. - PubMed
-
- Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. Valvano, S. Foster, T. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:652-654. - PubMed
-
- Chen, C., Y. Gong, M. Zhang, and J. Chen. 2004. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J. Biol. Chem. 279:25876-25882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

