ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes
- PMID: 17118980
- PMCID: PMC1828513
- DOI: 10.1128/IAI.01570-06
ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes
Abstract
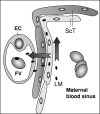
The facultative intracellular bacterial pathogen Listeria monocytogenes induces severe fetal infection during pregnancy. Little is known about the molecular mechanisms allowing the maternofetal transmission of bacteria. In this work, we studied fetoplacental invasion by infecting mice with various mutants lacking virulence factors involved in the intracellular life cycle of L. monocytogenes. We found that the placenta was highly susceptible to bacteria, including avirulent bacteria, such as an L. monocytogenes mutant with an hly deletion (DeltaLLO) and a nonpathogenic species, Listeria innocua, suggesting that permissive trophoblastic cells, trapping bacteria, provide a protective niche for bacterial survival. The DeltaLLO mutant, which is unable to escape the phagosomal compartment of infected cells, failed to grow in the trophoblast tissue and to invade the fetus. Mutant bacteria with inlA and inlB deletion (DeltaInlAB) grew in the placenta and fetus as well as did the wild-type virulent stain (EGDwt), indicating that in the murine model, internalins A and B are not involved in fetoplacental invasion by L. monocytogenes. Pregnant mice were then infected with an actA deletion (DeltaActA) strain, a virulence-attenuated mutant that is unable to polymerize actin and to spread from cell to cell. With the DeltaActA mutant, fetal infection occurs, but with a significant delay and restriction, and it requires a placental bacterial load 2 log units higher than that for the wild-type virulent strain. Definitive evidence for the role of ActA was provided by showing that a actA-complemented DeltaActA mutant was restored in its capacity to invade fetuses. ActA-mediated cell-to-cell spreading plays a major role in the vertical transmission of L. monocytogenes to the fetus in the murine model.
Figures

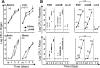


Similar articles
-
Cardiotropic Isolates of Listeria monocytogenes with Enhanced Vertical Transmission Dependent upon the Bacterial Surface Protein InlB.Infect Immun. 2021 Jan 19;89(2):e00321-20. doi: 10.1128/IAI.00321-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33139387 Free PMC article.
-
The pathogenesis of infection by Listeria monocytogenes.Microbiologia. 1996 Jun;12(2):245-58. Microbiologia. 1996. PMID: 8767708 Review.
-
Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6152-7. doi: 10.1073/pnas.0401434101. Epub 2004 Apr 8. Proc Natl Acad Sci U S A. 2004. PMID: 15073336 Free PMC article.
-
Listeriosis in the pregnant guinea pig: a model of vertical transmission.Infect Immun. 2004 Jan;72(1):489-97. doi: 10.1128/IAI.72.1.489-497.2004. Infect Immun. 2004. PMID: 14688130 Free PMC article.
-
Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion.Microbes Infect. 2007 Aug;9(10):1167-75. doi: 10.1016/j.micinf.2007.05.004. Epub 2007 May 7. Microbes Infect. 2007. PMID: 17761447 Review.
Cited by
-
IL-10 produced by trophoblast cells inhibits phagosome maturation leading to profound intracellular proliferation of Salmonella enterica Typhimurium.Placenta. 2013 Sep;34(9):765-74. doi: 10.1016/j.placenta.2013.06.003. Epub 2013 Jul 5. Placenta. 2013. PMID: 23834952 Free PMC article.
-
Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure.J Appl Physiol (1985). 2010 May;108(5):1347-56. doi: 10.1152/japplphysiol.01392.2009. Epub 2010 Mar 11. J Appl Physiol (1985). 2010. PMID: 20223995 Free PMC article.
-
Concepts and mechanisms: crossing host barriers.Cold Spring Harb Perspect Med. 2013 Jul 1;3(7):a010090. doi: 10.1101/cshperspect.a010090. Cold Spring Harb Perspect Med. 2013. PMID: 23818514 Free PMC article. Review.
-
Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens.Cell Host Microbe. 2011 Jul 21;10(1):54-64. doi: 10.1016/j.chom.2011.06.005. Cell Host Microbe. 2011. PMID: 21767812 Free PMC article.
-
Listeriolysin O: A phagosome-specific cytolysin revisited.Cell Microbiol. 2019 Mar;21(3):e12988. doi: 10.1111/cmi.12988. Epub 2019 Jan 15. Cell Microbiol. 2019. PMID: 30511471 Free PMC article. Review.
References
-
- Alouf, J. E. 2000. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 290:351-356. - PubMed
-
- Amarante-Paffaro, A., G. S. Queiroz, S. T. Correa, B. Spira, and E. Bevilacqua. 2004. Phagocytosis as a potential mechanism for microbial defense of mouse placental trophoblast cells. Reproduction 128:207-218. - PubMed
-
- Bakardjiev, A. I., B. A. Stacy, and D. A. Portnoy. 2005. Growth of Listeria monocytogenes in the Guinea pig placenta and role of cell-to-cell spread in fetal infection. J. Infect. Dis. 191:1889-1897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

