The broadly insecticidal Photorhabdus luminescens toxin complex a (Tca): activity against the Colorado potato beetle, Leptinotarsa decemlineata, and sweet potato whitefly, Bemisia tabaci
- PMID: 17119614
- PMCID: PMC1615239
- DOI: 10.1093/jis/5.1.32
The broadly insecticidal Photorhabdus luminescens toxin complex a (Tca): activity against the Colorado potato beetle, Leptinotarsa decemlineata, and sweet potato whitefly, Bemisia tabaci
Abstract
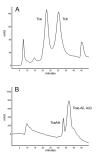
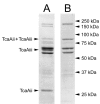
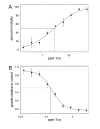
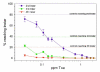
Toxin complex a (Tca), a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens, has been found to be orally toxic to both the Colorado potato beetle, Leptinotarsa decemlineata, and the sweet potato whitefly, Bemisia tabaci biotype B. The 48 hour LC50 for Tca against neonate L. decemlineata was found to be 2.7 ppm, and the growth of 2nd instar L. decemlineata exposed to Tca for 72 hours was almost entirely inhibited at concentrations above 0.5 ppm. B. tabaci was highly susceptible to Tca as well; newly emerged nymphs that were artificially fed Tca developed poorly, or not at all. Tca concentrations between 0.1 and 0.2 ppm reduced the number of nymphs reaching the second instar by 50%. In addition, a preparation of Tca missing two prominent subunits, TcaAii and TcaAiii, was found to be at least as toxic to L. decemlineata and B. tabaci as Tca itself, indicating that the activity of Tca is not dependant on the presence of these subunits at the time of ingestion.
Figures



References
-
- Bowen D, Rocheleau TA, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant RH. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. - PubMed
-
- Carazzi D. Eine neue Haematoxylinloesung. Zeitschrift fuer Wissenschaftliche Mikroskopie und fuer Mikroscopische Technik. 1911;28:273.
-
- Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles JF, Dassa E, Derose R, Derzelle S, Freyssinet G, Gaudriault S, Medigue C, Lanois A, Powell K, Siguier P, Vincent R, Wingate V, Zouine M, Glaser P, Boemare N, Danchin A, Kunst F. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nature Biotechnology. 2003;21:1307–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

