The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration?
- PMID: 17120192
- PMCID: PMC3901523
- DOI: 10.1002/bies.20502
The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration?
Abstract
The inner ear of mammals uses neurosensory cells derived from the embryonic ear for mechanoelectric transduction of vestibular and auditory stimuli (the hair cells) and conducts this information to the brain via sensory neurons. As with most other neurons of mammals, lost hair cells and sensory neurons are not spontaneously replaced and result instead in age-dependent progressive hearing loss. We review the molecular basis of neurosensory development in the mouse ear to provide a blueprint for possible enhancement of therapeutically useful transformation of stem cells into lost neurosensory cells. We identify several readily available adult sources of stem cells that express, like the ectoderm-derived ear, genes known to be essential for ear development. Use of these stem cells combined with molecular insights into neurosensory cell specification and proliferation regulation of the ear, might allow for neurosensory regeneration of mammalian ears in the near future.
Figures
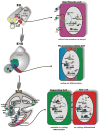
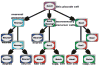

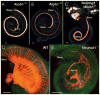

References
-
- Rakic P. Neuroscience. No more cortical neurons for you. Science. 2006;313:928–929. - PubMed
-
- Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, et al. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–191. - PubMed
-
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. - PubMed
-
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

