Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection
- PMID: 17121450
- PMCID: PMC1637124
- DOI: 10.1371/journal.pmed.0030441
Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection
Abstract
Background: To explore the possibility that antibody-mediated complement lysis contributes to viremia control in HIV-1 infection, we measured the activity of patient plasma in mediating complement lysis of autologous primary virus.
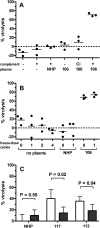
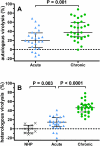
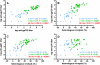
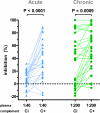
Methods and findings: Sera from two groups of patients-25 with acute HIV-1 infection and 31 with chronic infection-were used in this study. We developed a novel real-time PCR-based assay strategy that allows reliable and sensitive quantification of virus lysis by complement. Plasma derived at the time of virus isolation induced complement lysis of the autologous virus isolate in the majority of patients. Overall lysis activity against the autologous virus and the heterologous primary virus strain JR-FL was higher at chronic disease stages than during the acute phase. Most strikingly, we found that plasma virus load levels during the acute but not the chronic infection phase correlated inversely with the autologous complement lysis activity. Antibody reactivity to the envelope (Env) proteins gp120 and gp41 were positively correlated with the lysis activity against JR-FL, indicating that anti-Env responses mediated complement lysis. Neutralization and complement lysis activity against autologous viruses were not associated, suggesting that complement lysis is predominantly caused by non-neutralizing antibodies.
Conclusions: Collectively our data provide evidence that antibody-mediated complement virion lysis develops rapidly and is effective early in the course of infection; thus it should be considered a parameter that, in concert with other immune functions, steers viremia control in vivo.
Conflict of interest statement
Figures

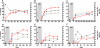


References
-
- Parren PW, Moore JP, Burton DR, Sattentau QJ. The neutralizing antibody response to HIV-1: Viral evasion and escape from humoral immunity. AIDS. 1999;13:S137–S162. - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207. - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

