Computational inference of neural information flow networks
- PMID: 17121460
- PMCID: PMC1664702
- DOI: 10.1371/journal.pcbi.0020161
Computational inference of neural information flow networks
Abstract
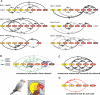
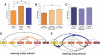
Determining how information flows along anatomical brain pathways is a fundamental requirement for understanding how animals perceive their environments, learn, and behave. Attempts to reveal such neural information flow have been made using linear computational methods, but neural interactions are known to be nonlinear. Here, we demonstrate that a dynamic Bayesian network (DBN) inference algorithm we originally developed to infer nonlinear transcriptional regulatory networks from gene expression data collected with microarrays is also successful at inferring nonlinear neural information flow networks from electrophysiology data collected with microelectrode arrays. The inferred networks we recover from the songbird auditory pathway are correctly restricted to a subset of known anatomical paths, are consistent with timing of the system, and reveal both the importance of reciprocal feedback in auditory processing and greater information flow to higher-order auditory areas when birds hear natural as opposed to synthetic sounds. A linear method applied to the same data incorrectly produces networks with information flow to non-neural tissue and over paths known not to exist. To our knowledge, this study represents the first biologically validated demonstration of an algorithm to successfully infer neural information flow networks.
Conflict of interest statement
Figures



References
-
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78.
-
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. - PubMed
-
- Gerstein GL, Bedenbaugh P, Aertsen MH. Neuronal assemblies. IEEE Trans Biomed Eng. 1989;36:4–14. - PubMed
-
- Freiwald WA, Valdes P, Bosch J, Biscay R, Jimenez JC, et al. Testing non-linearity and directedness of interactions between neural groups in the macaque inferotemporal cortex. J Neurosci Methods. 1999;94:105–119. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

