SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans
- PMID: 17121461
- PMCID: PMC1626103
- DOI: 10.1371/journal.ppat.0020110
SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans
Abstract
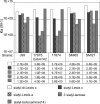
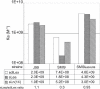
Adherence of Helicobacter pylori to inflamed gastric mucosa is dependent on the sialic acid-binding adhesin (SabA) and cognate sialylated/fucosylated glycans on the host cell surface. By in situ hybridization, H. pylori bacteria were observed in close association with erythrocytes in capillaries and post-capillary venules of the lamina propria of gastric mucosa in both infected humans and Rhesus monkeys. In vivo adherence of H. pylori to erythrocytes may require molecular mechanisms similar to the sialic acid-dependent in vitro agglutination of erythrocytes (i.e., sialic acid-dependent hemagglutination). In this context, the SabA adhesin was identified as the sialic acid-dependent hemagglutinin based on sialidase-sensitive hemagglutination, binding assays with sialylated glycoconjugates, and analysis of a series of isogenic sabA deletion mutants. The topographic presentation of binding sites for SabA on the erythrocyte membrane was mapped to gangliosides with extended core chains. However, receptor mapping revealed that the NeuAcalpha2-3Gal-disaccharide constitutes the minimal sialylated binding epitope required for SabA binding. Furthermore, clinical isolates demonstrated polymorphism in sialyl binding and complementation analysis of sabA mutants demonstrated that polymorphism in sialyl binding is an inherent property of the SabA protein itself. Gastric inflammation is associated with periodic changes in the composition of mucosal sialylation patterns. We suggest that dynamic adaptation in sialyl-binding properties during persistent infection specializes H. pylori both for individual variation in mucosal glycosylation and tropism for local areas of inflamed and/or dysplastic tissue.
Conflict of interest statement
Competing interests. In 2002 TB filed a patent application for the use of SabA as a vaccine candidate, International PCT pending number PCT/SE02/00301/ Helicobacter pylori sialic acid binding adhesin, SabA, and sabA gene.
Figures





References
-
- Cover TL, Berg DE, Blaser MJ, Mobley HLT. Helicobacter pylori pathogenesis. In: Groisman EA, editor. Principles of bacterial pathogenesis. San Diego (California): Academic Press; 2001. pp. 509–558.
-
- Alm RA, Ling LS, Moir DT, King BL, Brown ED, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. - PubMed
-
- Emody L, Carlsson A, Ljungh A, Wadström T. Mannose-resistant haemagglutination by Campylobacter pylori. Scand J Infect Dis. 1988;20:353–354. - PubMed
-
- Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Borén T, et al. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem. 2005;280:15390–15397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

