E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein
- PMID: 17121805
- PMCID: PMC1797514
- DOI: 10.1128/JVI.01712-06
E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein
Abstract
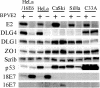
In most cervical cancers, DNAs of high-risk mucosotropic human papillomaviruses (HPVs), such as types 16 and 18, are maintained so as to express two viral proteins, E6 and E7, suggesting that they play important roles in carcinogenesis. The carboxy-terminal PDZ domain-binding motif of the E6 proteins is in fact essential for transformation of rodent cells and induction of hyperplasia in E6-transgenic mouse skin. To date, seven PDZ domain-containing proteins, including DLG1/hDLG, which is a human homologue of the Drosophila discs large tumor suppressor (Dlg), have been identified as targets of high-risk HPV E6 proteins. Here, we describe DLG4/PSD95, another human homologue of Dlg, as a novel E6 target. DLG4 was found to be expressed in normal human cells, including cervical keratinocytes, but only to a limited extent in both HPV-positive and HPV-negative cervical cancer cell lines. Expression of HPV18 E6 in HCK1T decreased DLG4 levels more strongly than did HPV16 E6, the carboxy-terminal motif of the proteins being critical for binding and degradation of DLG4 in vitro. DLG4 levels were restored by expression of either E6AP-specific short hairpin RNA or bovine papillomavirus type 1 E2 in HeLa but not CaSki or SiHa cells, reflecting downregulation of DLG4 mRNA as opposed to protein by an HPV-independent mechanism in HPV16-positive cancer lines. The tumorigenicity of CaSki cells was strongly inhibited by forced expression of DLG4, while growth in culture was not inhibited at all. These results suggest that DLG4 may function as a tumor suppressor in the development of HPV-associated cancers.
Figures






References
-
- Christopherson, K. S., B. J. Hillier, W. A. Lim, and D. S. Bredt. 1999. PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 274:27467-27473. - PubMed
-
- Dostatni, N., P. F. Lambert, R. Sousa, J. Ham, P. M. Howley, and M. Yaniv. 1991. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 5:1657-1671. - PubMed
-
- El-Husseini, A. E., E. Schnell, D. M. Chetkovich, R. A. Nicoll, and D. S. Bredt. 2000. PSD-95 involvement in maturation of excitatory synapses. Science 290:1364-1368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

