IkappaB kinase subunits alpha and gamma are required for activation of NF-kappaB and induction of apoptosis by mammalian reovirus
- PMID: 17121808
- PMCID: PMC1797491
- DOI: 10.1128/JVI.01860-06
IkappaB kinase subunits alpha and gamma are required for activation of NF-kappaB and induction of apoptosis by mammalian reovirus
Abstract
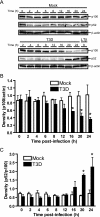
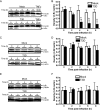
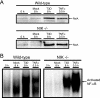
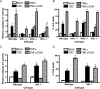
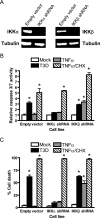
Reoviruses induce apoptosis both in cultured cells and in vivo. Apoptosis plays a major role in the pathogenesis of reovirus encephalitis and myocarditis in infected mice. Reovirus-induced apoptosis is dependent on the activation of transcription factor NF-kappaB and downstream cellular genes. To better understand the mechanism of NF-kappaB activation by reovirus, NF-kappaB signaling intermediates under reovirus control were investigated at the level of Rel, IkappaB, and IkappaB kinase (IKK) proteins. We found that reovirus infection leads initially to nuclear translocation of p50 and RelA, followed by delayed mobilization of c-Rel and p52. This biphasic pattern of Rel protein activation is associated with the degradation of the NF-kappaB inhibitor IkappaBalpha but not the structurally related inhibitors IkappaBbeta or IkappaBepsilon. Using IKK subunit-specific small interfering RNAs and cells deficient in individual IKK subunits, we demonstrate that IKKalpha but not IKKbeta is required for reovirus-induced NF-kappaB activation and apoptosis. Despite the preferential usage of IKKalpha, both NF-kappaB activation and apoptosis were attenuated in cells lacking IKKgamma/Nemo, an essential regulatory subunit of IKKbeta. Moreover, deletion of the gene encoding NF-kappaB-inducing kinase, which is known to modulate IKKalpha function, had no inhibitory effect on either response in reovirus-infected cells. Collectively, these findings indicate a novel pathway of NF-kappaB/Rel activation involving IKKalpha and IKKgamma/Nemo, which together mediate the expression of downstream proapoptotic genes in reovirus-infected cells.
Figures



Similar articles
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis.J Exp Med. 1999 Jun 7;189(11):1839-45. doi: 10.1084/jem.189.11.1839. J Exp Med. 1999. PMID: 10359587 Free PMC article.
-
NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha.Infect Immun. 2005 Jan;73(1):155-65. doi: 10.1128/IAI.73.1.155-165.2005. Infect Immun. 2005. PMID: 15618150 Free PMC article.
-
Interleukin-1-induced NF-kappaB activation is NEMO-dependent but does not require IKKbeta.J Biol Chem. 2007 Mar 23;282(12):8724-33. doi: 10.1074/jbc.M609613200. Epub 2007 Jan 23. J Biol Chem. 2007. PMID: 17244613 Free PMC article.
-
Extending the nuclear roles of IkappaB kinase subunits.Biochem Pharmacol. 2006 Oct 30;72(9):1081-9. doi: 10.1016/j.bcp.2006.06.017. Epub 2006 Jul 18. Biochem Pharmacol. 2006. PMID: 16846590 Review.
Cited by
-
Differential Delivery of Genomic Double-Stranded RNA Causes Reovirus Strain-Specific Differences in Interferon Regulatory Factor 3 Activation.J Virol. 2018 Apr 13;92(9):e01947-17. doi: 10.1128/JVI.01947-17. Print 2018 May 1. J Virol. 2018. PMID: 29437975 Free PMC article.
-
Noncanonical Cell Death Induction by Reassortant Reovirus.J Virol. 2020 Oct 27;94(22):e01613-20. doi: 10.1128/JVI.01613-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847857 Free PMC article.
-
Effects of resveratrol and its derivative pterostilbene on hepatic injury and immunological stress of weaned piglets challenged with lipopolysaccharide.J Anim Sci. 2022 Dec 1;100(12):skac339. doi: 10.1093/jas/skac339. J Anim Sci. 2022. PMID: 36242589 Free PMC article.
-
Modulation of NF-κB signalling by microbial pathogens.Nat Rev Microbiol. 2011 Apr;9(4):291-306. doi: 10.1038/nrmicro2539. Epub 2011 Mar 8. Nat Rev Microbiol. 2011. PMID: 21383764 Free PMC article. Review.
-
Bid regulates the pathogenesis of neurotropic reovirus.PLoS Pathog. 2010 Jul 1;6(7):e1000980. doi: 10.1371/journal.ppat.1000980. PLoS Pathog. 2010. PMID: 20617182 Free PMC article.
References
-
- Anest, V., J. L. Hanson, P. C. Cogswell, K. A. Steinbrecher, B. D. Strahl, and A. S. Baldwin. 2003. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature 423:659-663. - PubMed
-
- Baeuerle, P., and D. Baltimore. 1989. A 65-kD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes Dev. 3:1689-1698. - PubMed
-
- Baeuerle, P., and D. Baltimore. 1988. IκB: a specific inhibitor of the NF-κB transcription factor. Science 242:540-546. - PubMed
-
- Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. - PubMed
-
- Bowie, A. G., J. Zhan, and W. L. Marshall. 2004. Viral appropriation of apoptotic and NF-κB signaling pathways. J. Cell. Biochem. 91:1099-1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA82556/CA/NCI NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- T32 CA009385/CA/NCI NIH HHS/United States
- R01 AI052379/AI/NIAID NIH HHS/United States
- R01 AI52379/AI/NIAID NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA082556/CA/NCI NIH HHS/United States
- R01 AI50080/AI/NIAID NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 AI050080/AI/NIAID NIH HHS/United States
- R01 GM066882/GM/NIGMS NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- T32 CA09385/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

