Multiple valence superatoms
- PMID: 17121987
- PMCID: PMC1693677
- DOI: 10.1073/pnas.0608781103
Multiple valence superatoms
Abstract
We recently demonstrated that, in gas phase clusters containing aluminum and iodine atoms, an Al(13) cluster behaves like a halogen atom, whereas an Al(14) cluster exhibits properties analogous to an alkaline earth atom. These observations, together with our findings that Al(13)(-) is inert like a rare gas atom, have reinforced the idea that chosen clusters can exhibit chemical behaviors reminiscent of atoms in the periodic table, offering the exciting prospect of a new dimension of the periodic table formed by cluster elements, called superatoms. As the behavior of clusters can be controlled by size and composition, the superatoms offer the potential to create unique compounds with tailored properties. In this article, we provide evidence of an additional class of superatoms, namely Al(7)(-), that exhibit multiple valences, like some of the elements in the periodic table, and hence have the potential to form stable compounds when combined with other atoms. These findings support the contention that there should be no limitation in finding clusters, which mimic virtually all members of the periodic table.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

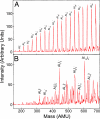
 ). The clusters are subsequently reacted with molecular oxygen at thermal energy.
). The clusters are subsequently reacted with molecular oxygen at thermal energy.


References
-
- Khanna SN, Jena P. Phys Rev Lett. 1992;69:1664–1667. - PubMed
-
- Bergeron DE, Castleman AW, Jr, Morisato T, Khanna SN. Science. 2004;304:84–87. - PubMed
-
- Bergeron DE, Roach PJ, Castleman AW, Jr, Jones NO, Khanna SN. Science. 2005;307:231–235. - PubMed
-
- Zhao J, Xie R-H. Phys Rev B. 2003;68:035401-1–035401-5.
-
- Zheng W, Nilles JM, Radisic D, Bowen KH. J Chem Phys. 2005;122:071101-1–071101-4. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

