Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression
- PMID: 17121991
- PMCID: PMC1693730
- DOI: 10.1073/pnas.0606298103
Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression
Abstract
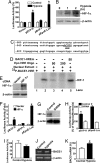
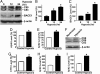
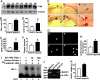
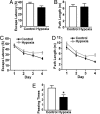
The molecular mechanism underlying the pathogenesis of the majority of cases of sporadic Alzheimer's disease (AD) is unknown. A history of stroke was found to be associated with development of some AD cases, especially in the presence of vascular risk factors. Reduced cerebral perfusion is a common vascular component among AD risk factors, and hypoxia is a direct consequence of hypoperfusion. Previously we showed that expression of the beta-site beta-amyloid precursor protein (APP) cleavage enzyme 1 (BACE1) gene BACE1 is tightly controlled at both the transcriptional and translational levels and that increased BACE1 maturation contributes to the AD pathogenesis in Down's syndrome. Here we have identified a functional hypoxia-responsive element in the BACE1 gene promoter. Hypoxia up-regulated beta-secretase cleavage of APP and amyloid-beta protein (Abeta) production by increasing BACE1 gene transcription and expression both in vitro and in vivo. Hypoxia treatment markedly increased Abeta deposition and neuritic plaque formation and potentiated the memory deficit in Swedish mutant APP transgenic mice. Taken together, our results clearly demonstrate that hypoxia can facilitate AD pathogenesis, and they provide a molecular mechanism linking vascular factors to AD. Our study suggests that interventions to improve cerebral perfusion may benefit AD patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Ann NY Acad Sci. 2002;977:9–23. - PubMed
-
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. J Am Med Assoc. 1997;277:813–817. - PubMed
-
- Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, Lenzi GL. Neurology. 2004;62:2193–2197. - PubMed
-
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Neurology. 2003;60:1082–1088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

