PKB and megalin determine the survival or death of renal proximal tubule cells
- PMID: 17121993
- PMCID: PMC1693744
- DOI: 10.1073/pnas.0605029103
PKB and megalin determine the survival or death of renal proximal tubule cells
Abstract
Renal proximal tubule cells have a remarkable ability to reabsorb large quantities of albumin through megalin-mediated endocytosis. This is an essential process for overall body homeostasis. Overstressing this endocytic system with a prolonged excess of albumin is injurious to proximal tubule cells. How these cells function and protect themselves from injury is unknown. Here, we show that megalin is the sensor that determines whether cells will be protected or injured by albumin. Megalin, through a novel mechanism, binds PKB in a D-3-phosphorylated phospholipid-insensitive manner, anchoring PKB in the luminal plasma membrane. Whereas low doses of albumin are protective, an overload of albumin decreases megalin expression followed by a reduction of plasma membrane PKB, PKB activity, and Bad phosphorylation induced by PKB. The result is albumin-induced apoptosis. These results reveal a model for PKB distribution in the plasma membrane and elucidate mechanisms involved in both the protective and toxic effects of albumin on proximal tubule cells. In addition, our findings suggest a mechanism for the progression of chronic kidney disease to end-stage renal disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
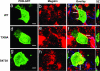


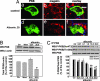

References
-
- Birn H, Christensen EI. Kidney Int. 2006;69:440–449. - PubMed
-
- D'Amico G, Bazzi C. Kidney Int. 2003;63:809–825. - PubMed
-
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. J Am Soc Nephrol. 1998;9:960–968. - PubMed
-
- Ruggenenti P, Remuzzi G. Annu Rev Med. 2000;51:315–327. - PubMed
-
- Zoja C, Morigi M, Remuzzi G. J Am Soc Nephrol. 2003;14:S36–S41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

