Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain
- PMID: 17122038
- PMCID: PMC6675439
- DOI: 10.1523/JNEUROSCI.2783-06.2006
Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain
Abstract
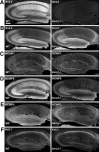
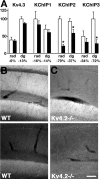
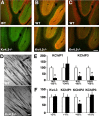
Kv4 family voltage-gated potassium channel alpha subunits and Kv channel-interacting protein (KChIP) and dipeptidyl aminopeptidase-like protein subunits comprise somatodendritic A-type channels in mammalian neurons. Recently, a mouse was generated with a targeted deletion of Kv4.2, a Kv4 alpha subunit expressed in many but not all mammalian brain neurons. Kv4.2-/- mice are grossly indistinguishable from wild-type (WT) littermates. Here we used immunohistochemistry to analyze expression of component Kv4 and KChIP subunits of A-type channels in WT and Kv4.2-/- brains. We found that the expression level, and cellular and subcellular distribution of the other prominent brain Kv4 family member Kv4.3, was indistinguishable between WT and Kv4.2-/- samples. However, we found unanticipated regional and cell-specific decreases in expression of KChIPs. The degree of altered expression of individual KChIP isoforms in different regions and neurons precisely follows the level of Kv4.2 normally found at those sites and presumably their extent of association of these KChIPs with Kv4.2. The dramatic effects of Kv4.2 deletion on KChIP expression suggest that, in addition to previously characterized effects of KChIPs on the functional properties, trafficking, and turnover rate of Kv4 channels, Kv4:KChIP association may confer reciprocal Kv4.2-dependent effects on KChIPs. The impact of Kv4.2 deletion on KChIP expression also supports the major role of KChIPs as auxiliary subunits of Kv4 channels.
Figures
References
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35:851–865. - PubMed
-
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. - PubMed
-
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
