Non-Gaussian membrane potential dynamics imply sparse, synchronous activity in auditory cortex
- PMID: 17122045
- PMCID: PMC6675435
- DOI: 10.1523/JNEUROSCI.2813-06.2006
Non-Gaussian membrane potential dynamics imply sparse, synchronous activity in auditory cortex
Abstract
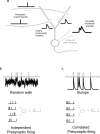
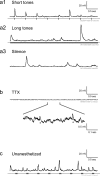
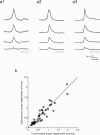
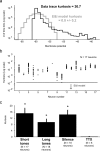
Many models of cortical dynamics have focused on the high-firing regime, in which neurons are driven near their maximal rate. Here we consider the responses of neurons in auditory cortex under typical low-firing rate conditions, when stimuli have not been optimized to drive neurons maximally. We used whole-cell patch-clamp recording in vivo to measure subthreshold membrane potential fluctuations in rat primary auditory cortex in both the anesthetized and awake preparations. By analyzing the subthreshold membrane potential dynamics on single trials, we made inferences about the underlying population activity. We found that, during both spontaneous and evoked responses, membrane potential was highly non-Gaussian, with dynamics consisting of occasional large excursions (sometimes tens of millivolts), much larger than the small fluctuations predicted by most random walk models that predict a Gaussian distribution of membrane potential. Thus, presynaptic inputs under these conditions are organized into quiescent periods punctuated by brief highly synchronous volleys, or "bumps." These bumps were typically so brief that they could not be well characterized as "up states" or "down states." We estimate that hundreds, perhaps thousands, of presynaptic neurons participate in the largest volleys. These dynamics suggest a computational scheme in which spike timing is controlled by concerted firing among input neurons rather than by small fluctuations in a sea of background activity.
Figures



References
-
- Aertsen A, Diesmann M, Gewaltig MO. Propagation of synchronous spiking activity in feedforward neural networks. J Physiol (Paris) 1996;90:243–247. - PubMed
-
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat Neurosci. 2000;3:617–621. - PubMed
-
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–1237. - PubMed
-
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput. 1996;8:1185–1202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
