Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis
- PMID: 17122345
- PMCID: PMC1797294
- DOI: 10.1128/JB.00845-06
Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis
Abstract
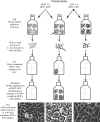
Genetic recombinants that resulted from lateral gene transfer (LGT) have been detected in sexually transmitted disease isolates of Chlamydia trachomatis, but a mechanism for LGT in C. trachomatis has not been described. We describe here a system that readily detects C. trachomatis LGT in vitro and that may facilitate discovery of its mechanisms. Host cells were simultaneously infected in the absence of antibiotics with an ofloxacin-resistant mutant and a second mutant that was resistant to lincomycin, trimethoprim, or rifampin. Selection for doubly resistant C. trachomatis isolates in the progeny detected apparent recombinant frequencies of 10(-4) to 10(-3), approximately 10(4) times more frequent than doubly resistant spontaneous mutants in progeny from uniparental control infections. Polyclonal doubly resistant populations and clones isolated from them in the absence of antibiotics had the specific resistance-conferring mutations present in the parental mutants; absence of the corresponding normal nucleotides indicated that they had been replaced by homologous recombination. These results eliminate spontaneous mutation, between-strain complementation, and heterotypic resistance as general explanations of multiply resistant C. trachomatis that originated in mixed infections in our experiments and demonstrate genetic stability of the recombinants. The kind of LGT we observed might be useful for creating new strains for functional studies by creating new alleles or combinations of alleles of polymorphic loci and might also disseminate antibiotic resistance genes in vivo. The apparent absence of phages and conjugative plasmids in C. trachomatis suggests that the LGT may have occurred by means of natural DNA transformation. Therefore, the experimental system may have implications for genetically altering C. trachomatis by means of DNA transfer.
Figures


References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., Hoboken, NJ.
-
- Barnes, R. C., R. J. Suchland, S. P. Wang, C. C. Kuo, and W. E. Stamm. 1985. Detection of multiple serovars of Chlamydia trachomatis in genital infections. J. Infect. Dis. 152:985-989. - PubMed
-
- Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

