Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell
- PMID: 17123568
- PMCID: PMC1885464
- DOI: 10.1016/j.virol.2006.10.025
Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell
Abstract
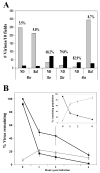
GFP-Vpr labeled HIV-1 virions have provided a method to visually examine the interactions between the virus and target cell during infection. However, existing methods to discriminate between virions that have been non-specifically endocytosed from those that have productively entered the host cell cytoplasm have remained problematic. Therefore, we examined the ability of a series of membrane-targeted fluorescent fusion protein constructs to be incorporated into virions. We find that a fluorescent protein fusion targeted to the plasma membrane by the addition of the N-terminal 15 amino acid sequence of c-Src (S15) is efficiently packaged into HIV virions. Using fluorescent proteins fused to this sequence, we have generated virions dually labeled with S15-mCherry and GFP-Vpr. Importantly, we can detect the loss of this S15-mCherry membrane signal following fusion. After infection with VSV-g pseudotyped HIV virions, we find a measurable, specific loss of membrane label during infection. This loss of fluorescence is not observed when fusion is prevented using bafilomycin A. This increased ability to discriminate between non-productively endocytosed virions and those actively undergoing steps in the infectious process will facilitate efforts to examine early steps in infection microscopically.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

