Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response
- PMID: 17123686
- PMCID: PMC1945101
- DOI: 10.1016/j.tox.2006.10.014
Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response
Abstract
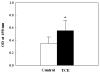
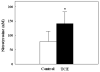
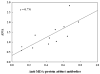
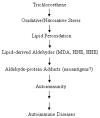
Reactive oxygen and nitrogen species (RONS) are implicated in the pathogenesis of several autoimmune diseases. Also, increased lipid peroxidation and protein nitration are reported in systemic autoimmune diseases. Lipid peroxidation-derived aldehydes (LPDAs) such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are highly reactive and bind proteins covalently, but their potential to elicit an autoimmune response and contribution to disease pathogenesis remain unclear. Similarly, nitration of protein could also contribute to disease pathogenesis. To assess the status of lipid peroxidation and/or RONS, autoimmune-prone female MRL+/+ mice (5-week old) were treated with trichloroethene (TCE), an environmental contaminant known to induce autoimmune response, for 48 weeks (0.5mg/ml via drinking water), and formation of antibodies to LPDA-protein adducts was followed in the sera of control and TCE-treated mice. TCE treatment led to greater formation of both anti-MDA- and -HNE-protein adduct antibodies and higher serum iNOS and nitrotyrosine levels. The increase in TCE-induced oxidative stress was associated with increases in anti-nuclear-, anti-ssDNA- and anti-dsDNA-antibodies. These findings suggest that TCE exposure not only leads to oxidative/nitrosative stress, but is also associated with induction/exacerbation of autoimmune response in MRL+/+ mice. Further interventional studies are needed to establish a causal role of RONS in TCE-mediated autoimmunity.
Figures






References
-
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. - PubMed
-
- Biemond P, Swaak AJ, Koster JF. Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1984;27:760–765. - PubMed
-
- Channel SR, Latendresse JR, Kidney JK, Grabau JH, Lane JW, Steel-Goodwin L, Gothaus MC. A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol Sci. 1998;43:145–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

