Presentation in relation to publication of results from clinical trials
- PMID: 17123868
- PMCID: PMC2946790
- DOI: 10.1016/j.cct.2006.10.005
Presentation in relation to publication of results from clinical trials
Abstract
Background: Results from clinical trials are typically disseminated first by presentation at scientific meetings. An important question has to do with the role of presentation in improving the quality of manuscripts submitted to the journals as well as the effect of presentation in speeding, or delaying subsequent publication. The aim of this research is focused on presentation practices of trialists to examine their effect on the timing of publications of clinical trial results.
Methods: Six hundred and one (601) trials published in 1996 and 1997 were identified via MEDLINE using medical subject heading "clinical trials" or the occurrence of the term in the text and by limiting to publication type "clinical trial". Authors of those trials were surveyed to determine prior presentation history for the identified trials.
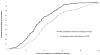
Results: Among the 601 trials identified, complete responses to questionnaires were obtained for 379 (63%) trials. The median time from completion to first submission of the primary results manuscript was 11 months and the median time from completion to publication was 25 months for the 220 trials involving presentation prior to submission for publication. The corresponding median times from completion to first submission and publication for the subset of trials not involving presentation prior to the submission were 8 and 19 months (159 trials), respectively. The adjusted relative hazard for publication for trials involving presentation prior to first submission was 0.55 versus trials not involving presentation prior to first submission (95% confidence interval, 0.44 to 0.69).
Conclusion: Despite the importance of dissemination of results prior to publication, investigators should carefully weigh a potential gain in quality against a potential for delay in submission of the primary results manuscript by presentation at scientific meetings. The findings of our study suggest that presentation prior to submission may increase time to publication. Inclusion of presentation dates in clinical trial registers should be considered to allow future studies investigating presentation and publication practices.
Figures
References
-
- Dudley HA. Surgical research: Master or servant. Am J Surg. 1978;135:458–460. - PubMed
-
- Landry VL. The publication outcome for the papers presented at the 1990 ABA conference. J Burn Care Rehabil. 1996;17:23A–26A. - PubMed
-
- Bowrey DB, Morris-Stiff GJ, Clark GWB, Carey PD, Mansel RE. Peer-reviewed publication following presentation at a regional surgical meeting. Med Educ. 1999;33:212–214. - PubMed
-
- Goldman L, Loscalzo A. Fate of cardiology research originally published in abstract form. N Engl J Med. 1980;303:255–259. - PubMed
-
- Meranze J, Ellison N, Greenhow E. Publications resulting from anesthesia meeting abstracts. Anesth Analg. 1982;61:445–448. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical