Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4
- PMID: 17124166
- PMCID: PMC1693708
- DOI: 10.1073/pnas.0609061103
Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4
Abstract
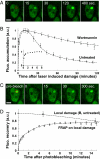
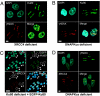
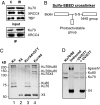
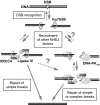
DNA double-strand break (DSB) repair by nonhomologous end joining (NHEJ) requires the assembly of several proteins on DNA ends. Although biochemical studies have elucidated several aspects of the NHEJ reaction mechanism, much less is known about NHEJ in living cells, mainly because of the inability to visualize NHEJ repair proteins at DNA damage. Here we provide evidence that a pulsed near IR laser can produce DSBs without any visible alterations in the nucleus, and we show that NHEJ proteins accumulate in the irradiated areas. The levels of DSBs and Ku accumulation diminished in time, showing that this approach allows us to study DNA repair kinetics in vivo. Remarkably, the Ku heterodimers on DNA ends were in dynamic equilibrium with Ku70/80 in solution, showing that NHEJ complex assembly is reversible. Accumulation of XRCC4/ligase IV on DSBs depended on the presence of Ku70/80, but not DNA-PK(CS). We detected a direct interaction between Ku70 and XRCC4 that could explain these requirements. Our results suggest that this assembly constitutes the core of the NHEJ reaction and that XRCC4 may serve as a flexible tether between Ku70/80 and ligase IV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

