NMDA receptor surface mobility depends on NR2A-2B subunits
- PMID: 17124177
- PMCID: PMC1693737
- DOI: 10.1073/pnas.0605238103
NMDA receptor surface mobility depends on NR2A-2B subunits
Abstract
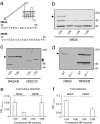
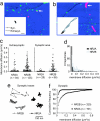
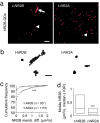
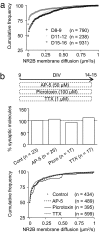
The NR2 subunit composition of NMDA receptors (NMDARs) varies during development, and this change is important in NMDAR-dependent signaling. In particular, synaptic NMDAR switch from containing mostly NR2B subunit to a mixture of NR2B and NR2A subunits. The pathways by which neurons differentially traffic NR2A- and NR2B-containing NMDARs are poorly understood. Using single-particle and -molecule approaches and specific antibodies directed against NR2A and NR2B extracellular epitopes, we investigated the surface mobility of native NR2A and NR2B subunits at the surface of cultured neurons. The surface mobility of NMDARs depends on the NR2 subunit subtype, with NR2A-containing NMDARs being more stable than NR2B-containing ones, and NR2A subunit overexpression stabilizes surface NR2B-containing NMDARs. The developmental change in the synaptic surface content of NR2A and NR2B subunits was correlated with a developmental change in the time spent by the subunits within synapses. This suggests that the switch in synaptic NMDAR subtypes depends on the regulation of the receptor surface trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Nature. 1994;368:144–147. - PubMed
-
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Annu Rev Pharmacol Toxicol. 2003;43:335–358. - PubMed
-
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Nature. 1984;307:462–465. - PubMed
-
- van Zundert B, Yoshii A, Constantine-Paton M. Trends Neurosci. 2004;27:428–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

