A Cdt1-geminin complex licenses chromatin for DNA replication and prevents rereplication during S phase in Xenopus
- PMID: 17124498
- PMCID: PMC1698883
- DOI: 10.1038/sj.emboj.7601436
A Cdt1-geminin complex licenses chromatin for DNA replication and prevents rereplication during S phase in Xenopus
Abstract
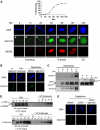
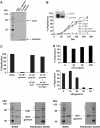
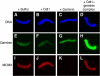
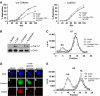
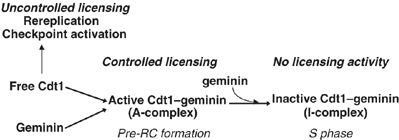
Initiation of DNA synthesis involves the loading of the MCM2-7 helicase onto chromatin by Cdt1 (origin licensing). Geminin is thought to prevent relicensing by binding and inhibiting Cdt1. Here we show, using Xenopus egg extracts, that geminin binding to Cdt1 is not sufficient to block its activity and that a Cdt1-geminin complex licenses chromatin, but prevents rereplication, working as a molecular switch at replication origins. We demonstrate that geminin is recruited to chromatin already during licensing, while bulk geminin is recruited at the onset of S phase. A recombinant Cdt1-geminin complex binds chromatin, interacts with the MCM2-7 complex and licenses chromatin once per cell cycle. Accordingly, while recombinant Cdt1 induces rereplication in G1 or G2 and activates an ATM/ATR-dependent checkpoint, the Cdt1-geminin complex does not. We further demonstrate that the stoichiometry of the Cdt1-geminin complex regulates its activity. Our results suggest a model in which the MCM2-7 helicase is loaded onto chromatin by a Cdt1-geminin complex, which is inactivated upon origin firing by binding additional geminin. This origin inactivation reaction does not occur if only free Cdt1 is present on chromatin.
Figures
Similar articles
-
Cell cycle regulation of the licensing activity of Cdt1 in Xenopus laevis.Exp Cell Res. 2004 Apr 15;295(1):138-49. doi: 10.1016/j.yexcr.2003.11.018. Exp Cell Res. 2004. PMID: 15051497
-
Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopus egg extracts.Genes Dev. 2005 Jan 1;19(1):114-26. doi: 10.1101/gad.1255805. Epub 2004 Dec 14. Genes Dev. 2005. PMID: 15598982 Free PMC article.
-
Inter-origin cooperativity of geminin action establishes an all-or-none switch for replication origin licensing.Genes Cells. 2011 Apr;16(4):380-96. doi: 10.1111/j.1365-2443.2011.01501.x. Genes Cells. 2011. PMID: 21426446
-
Cdt1 and geminin in DNA replication initiation.Subcell Biochem. 2012;62:71-87. doi: 10.1007/978-94-007-4572-8_5. Subcell Biochem. 2012. PMID: 22918581 Review.
-
Cdt1 and geminin: role during cell cycle progression and DNA damage in higher eukaryotes.Front Biosci. 2007 Jan 1;12:1629-41. doi: 10.2741/2175. Front Biosci. 2007. PMID: 17127409 Review.
Cited by
-
Knockdown of SCF(Skp2) function causes double-parked accumulation in the nucleus and DNA re-replication in Drosophila plasmatocytes.PLoS One. 2013 Oct 24;8(10):e79019. doi: 10.1371/journal.pone.0079019. eCollection 2013. PLoS One. 2013. PMID: 24205363 Free PMC article.
-
Cell cycle regulation of DNA replication.Annu Rev Genet. 2007;41:237-80. doi: 10.1146/annurev.genet.41.110306.130308. Annu Rev Genet. 2007. PMID: 17630848 Free PMC article. Review.
-
Dynamic association of ORCA with prereplicative complex components regulates DNA replication initiation.Mol Cell Biol. 2012 Aug;32(15):3107-20. doi: 10.1128/MCB.00362-12. Epub 2012 May 29. Mol Cell Biol. 2012. PMID: 22645314 Free PMC article.
-
Regulation of DNA Replication Licensing and Re-Replication by Cdt1.Int J Mol Sci. 2021 May 14;22(10):5195. doi: 10.3390/ijms22105195. Int J Mol Sci. 2021. PMID: 34068957 Free PMC article. Review.
-
Geminin Is Essential for Pluripotent Cell Viability During Teratoma Formation, but Not for Differentiated Cell Viability During Teratoma Expansion.Stem Cells Dev. 2017 Feb 15;26(4):285-302. doi: 10.1089/scd.2016.0260. Epub 2016 Nov 7. Stem Cells Dev. 2017. PMID: 27821018 Free PMC article.
References
-
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 - PubMed
-
- Benjamin JM, Torke SJ, Demeler B, McGarry TJ (2004) Geminin has dimerization, Cdt1-binding, and destruction domains that are required for biological activity. J Biol Chem 279: 45957–45984 - PubMed
-
- Blow JJ, Laskey RA (1988) A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332: 546–548 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

