Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release
- PMID: 17124501
- PMCID: PMC1698877
- DOI: 10.1038/sj.emboj.7601425
Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release
Abstract
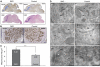
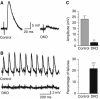
Alpha-RIMs (RIM1alpha and RIM2alpha) are multidomain active zone proteins of presynaptic terminals. Alpha-RIMs bind to Rab3 on synaptic vesicles and to Munc13 on the active zone via their N-terminal region, and interact with other synaptic proteins via their central and C-terminal regions. Although RIM1alpha has been well characterized, nothing is known about the function of RIM2alpha. We now show that RIM1alpha and RIM2alpha are expressed in overlapping but distinct patterns throughout the brain. To examine and compare their functions, we generated knockout mice lacking RIM2alpha, and crossed them with previously produced RIM1alpha knockout mice. We found that deletion of either RIM1alpha or RIM2alpha is not lethal, but ablation of both alpha-RIMs causes postnatal death. This lethality is not due to a loss of synapse structure or a developmental change, but to a defect in neurotransmitter release. Synapses without alpha-RIMs still contain active zones and release neurotransmitters, but are unable to mediate normal Ca(2+)-triggered release. Our data thus demonstrate that alpha-RIMs are not essential for synapse formation or synaptic exocytosis, but are required for normal Ca(2+)-triggering of exocytosis.
Figures






References
-
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N (2006) Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J Biol Chem 281: 19720–19731 - PubMed
-
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N (2001) Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron 30: 183–196 - PubMed
-
- Brose N, Hofmann K, Hata Y, Südhof TC (1995) Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem 270: 25273–25280 - PubMed
-
- Calakos N, Schoch S, Südhof TC, Malenka RC (2004) Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron 42: 889–896 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

