The supramolecular architecture of junctional microdomains in native lens membranes
- PMID: 17124511
- PMCID: PMC1796741
- DOI: 10.1038/sj.embor.7400858
The supramolecular architecture of junctional microdomains in native lens membranes
Abstract
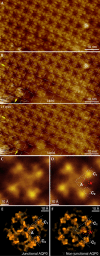
Gap junctions formed by connexons and thin junctions formed by lens-specific aquaporin 0 (AQP0) mediate the tight packing of fibre cells necessary for lens transparency. Gap junctions conduct water, ions and metabolites between cells, whereas junctional AQP0 seems to be involved in cell adhesion. High-resolution atomic force microscopy (AFM) showed the supramolecular organization of these proteins in native lens core membranes, in which AQP0 forms two-dimensional arrays that are surrounded by densely packed gap junction channels. These junctional microdomains simultaneously provide adhesion and communication between fibre cells. The AFM topographs also showed that the extracellular loops of AQP0 in junctional microdomains adopt a conformation that closely resembles the structure of junctional AQP0, in which the water pore is thought to be closed. Finally, time-lapse AFM imaging provided insights into AQP0 array formation. This first high-resolution view of a multicomponent eukaryotic membrane shows how membrane proteins self-assemble into functional microdomains.
Figures


References
-
- Agre P (2004) Nobel lecture. Aquaporin water channels. Biosci Rep 24: 127–163 - PubMed
-
- Alcala J, Lieska N, Maisel H (1975) Protein composition of bovine lens cortical fiber cell membranes. Exp Eye Res 21: 581–595 - PubMed
-
- Binnig G, Quate CF, Gerber C (1986) Atomic force microscope. Phys Rev Lett 56: 930–933 - PubMed
-
- Chandy G, Zampighi GA, Kreman M, Hall JE (1997) Comparison of the water transporting properties of MIP and AQP1. J Membr Biol 159: 29–39 - PubMed
-
- Donaldson P, Kistler J, Mathias RT (2001) Molecular solutions to mammalian lens transparency. News Physiol Sci 16: 118–123 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

