Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels
- PMID: 17124532
- PMCID: PMC1654201
- DOI: 10.1172/JCI26620
Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels
Abstract
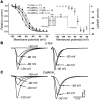
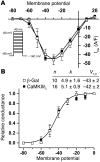
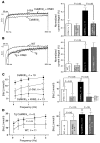
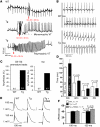
In heart failure (HF), Ca(2+)/calmodulin kinase II (CaMKII) expression is increased. Altered Na(+) channel gating is linked to and may promote ventricular tachyarrhythmias (VTs) in HF. Calmodulin regulates Na(+) channel gating, in part perhaps via CaMKII. We investigated effects of adenovirus-mediated (acute) and Tg (chronic) overexpression of cytosolic CaMKIIdelta(C) on Na(+) current (I(Na)) in rabbit and mouse ventricular myocytes, respectively (in whole-cell patch clamp). Both acute and chronic CaMKIIdelta(C) overexpression shifted voltage dependence of Na(+) channel availability by -6 mV (P < 0.05), and the shift was Ca(2+) dependent. CaMKII also enhanced intermediate inactivation and slowed recovery from inactivation (prevented by CaMKII inhibitors autocamtide 2-related inhibitory peptide [AIP] or KN93). CaMKIIdelta(C) markedly increased persistent (late) inward I(Na) and intracellular Na(+) concentration (as measured by the Na(+) indicator sodium-binding benzofuran isophthalate [SBFI]), which was prevented by CaMKII inhibition in the case of acute CaMKIIdelta(C) overexpression. CaMKII coimmunoprecipitates with and phosphorylates Na(+) channels. In vivo, transgenic CaMKIIdelta(C) overexpression prolonged QRS duration and repolarization (QT intervals), decreased effective refractory periods, and increased the propensity to develop VT. We conclude that CaMKII associates with and phosphorylates cardiac Na(+) channels. This alters I(Na) gating to reduce availability at high heart rate, while enhancing late I(Na) (which could prolong action potential duration). In mice, enhanced CaMKIIdelta(C) activity predisposed to VT. Thus, CaMKII-dependent regulation of Na(+) channel function may contribute to arrhythmogenesis in HF.
Figures



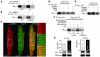
References
-
- Bennett P., Yazawa K., Makita N., George A. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. - PubMed
-
- Wang D.W., Makita N., Kitabatake A., Balser J.R., George A.L. Enhanced Na+ channel intermediate inactivation in Brugada syndrome. . Circ. Res. 2000;87:E37–E43. - PubMed
-
- Bers, D.M. 2001. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic Publishers. Dordrecht, The Netherlands. 427 pp.
-
- Tomaselli G.F., Zipes D.P. What causes sudden death in heart failure? Circ. Res. 2004;95:754–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

