CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo
- PMID: 17125495
- PMCID: PMC2000477
- DOI: 10.1186/jbiol50
CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo
Abstract
Background: Chemotherapy in cancer patients can be associated with serious short- and long-term adverse neurological effects, such as leukoencephalopathy and cognitive impairment, even when therapy is delivered systemically. The underlying cellular basis for these adverse effects is poorly understood.
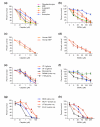
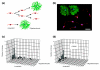
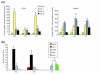
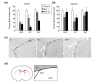
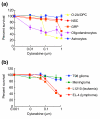
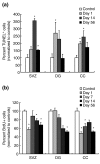
Results: We found that three mainstream chemotherapeutic agents--carmustine (BCNU), cisplatin, and cytosine arabinoside (cytarabine), representing two DNA cross-linking agents and an antimetabolite, respectively--applied at clinically relevant exposure levels to cultured cells are more toxic for the progenitor cells of the CNS and for nondividing oligodendrocytes than they are for multiple cancer cell lines. Enhancement of cell death and suppression of cell division were seen in vitro and in vivo. When administered systemically in mice, these chemotherapeutic agents were associated with increased cell death and decreased cell division in the subventricular zone, in the dentate gyrus of the hippocampus and in the corpus callosum of the CNS. In some cases, cell division was reduced, and cell death increased, for weeks after drug administration ended.
Conclusion: Identifying neural populations at risk during any cancer treatment is of great importance in developing means of reducing neurotoxicity and preserving quality of life in long-term survivors. Thus, as well as providing possible explanations for the adverse neurological effects of systemic chemotherapy, the strong correlations between our in vitro and in vivo analyses indicate that the same approaches we used to identify the reported toxicities can also provide rapid in vitro screens for analyzing new therapies and discovering means of achieving selective protection or targeted killing.
Figures








References
-
- Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. J Clin Oncol. 1994;12:2216–2228. - PubMed
-
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

