Nuclear Factor 90, a cellular dsRNA binding protein inhibits the HIV Rev-export function
- PMID: 17125513
- PMCID: PMC1713252
- DOI: 10.1186/1742-4690-3-83
Nuclear Factor 90, a cellular dsRNA binding protein inhibits the HIV Rev-export function
Abstract
Background: The HIV Rev protein is known to facilitate export of incompletely spliced and unspliced viral transcripts to the cytoplasm, a necessary step in virus life cycle. The Rev-mediated nucleo-cytoplasmic transport of nascent viral transcripts, dependents on interaction of Rev with the RRE RNA structural element present in the target RNAs. The C-terminal variant of dsRNA-binding nuclear protein 90 (NF90ctv) has been shown to markedly attenuate viral replication in stably transduced HIV-1 target cell line. Here we examined a mechanism of interference of viral life cycle involving Rev-NF90ctv interaction.
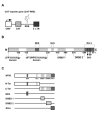
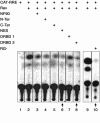
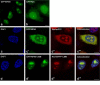
Results: Since Rev:RRE complex formations depend on protein:RNA and protein:protein interactions, we investigated whether the expression of NF90ctv might interfere with Rev-mediated export of RRE-containing transcripts. When HeLa cells expressed both NF90ctv and Rev protein, we observed that NF90ctv inhibited the Rev-mediated RNA transport. In particular, three regions of NF90ctv protein are involved in blocking Rev function. Moreover, interaction of NF90ctv with the RRE RNA resulted in the expression of a reporter protein coding sequences linked to the RRE structure. Moreover, Rev influenced the subcellular localization of NF90ctv, and this process is leptomycin B sensitive.
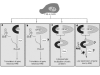
Conclusion: The dsRNA binding protein, NF90ctv competes with HIV Rev function at two levels, by competitive protein:protein interaction involving Rev binding to specific domains of NF90ctv, as well as by its binding to the RRE-RNA structure. Our results are consistent with a model of Rev-mediated HIV-1 RNA export that envisions Rev-multimerization, a process interrupted by NF90ctv.
Figures



References
-
- Kjems J, Askjaer P. Rev protein and its cellular partners. Adv Pharmacol. 2000;48:251–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

