Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach
- PMID: 17125526
- PMCID: PMC1794520
- DOI: 10.1186/ar2085
Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach
Abstract
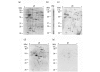
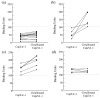
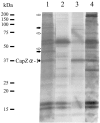
Recently, autoantibodies to some citrullinated autoantigens have been reported to be specific for rheumatoid arthritis (RA). However, an entire profile of and autoimmunity of the citrullinated proteins have been poorly understood. To understand the profile, we examined citrullinated autoantigens by a proteomic approach and further investigated the significance of citrullination in antigenicity of one of the autoantigens. Specifically, we detected citrullinated autoantigens in synovial tissue of a patient with RA by two-dimensional electrophoresis and Western blotting by using pooled sera from five patients with RA and anti-citrulline antibodies. After identifying the detected autoantigens by mass spectrometry, we investigated the contribution of citrullination to autoantigenicity by using a recombinant protein with or without citrullination on one of the identified novel citrullinated autoantigens. As a result, we found 51 citrullinated protein spots. Thirty (58.8%) of these spots were autoantigenic. We identified 13 out of the 30 detected citrullinated autoantigenic proteins. They contained three fibrinogen derivatives and several novel citrullinated autoantigens (for example, asporin and F-actin capping protein alpha-1 subunit [CapZalpha-1]). We further analyzed the contribution of citrullination to autoantigenicity in one of the detected citrullinated autoantigens, CapZalpha-1. As a result, frequencies of autoantibodies to non-citrullinated CapZalpha-1 were 36.7% in the RA group tested, 10.7% in the osteoarthritis (OA) group, and 6.5% in healthy donors. On the other hand, those to citrullinated CapZalpha-1 were 53.3% in the RA group, 7.1% in the OA group, and 6.5% in the healthy donors. This shows that autoantigenicity of citrullinated or non-citrullinated CapZalpha-1 is relevant to RA. The antibody titers to the citrullinated CapZalpha-1 were significantly higher than those to the non-citrullinated CapZalpha-1 in 36.7% of patients; however, the other patients showed almost equal antibody titers to both citrullinated and non-citrullinated CapZalpha-1. Therefore, the autoantibodies would target citrulline-related and/or citrulline-unrelated epitope(s) of CapZalpha-1. In conclusion, we report a profile of citrullinated autoantigens for the first time. Even though citrullination is closely related to autoantigenicity, citrullination would not always produce autoantigenicity in RA. Citrullinated and non-citrullinated autoantigens/autoepitopes would have different pathological roles in RA.
Figures



Similar articles
-
Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis.Arthritis Rheum. 2007 Aug;56(8):2503-11. doi: 10.1002/art.22817. Arthritis Rheum. 2007. PMID: 17665451
-
Citrullination of synovial proteins in murine models of rheumatoid arthritis.Arthritis Rheum. 2003 Sep;48(9):2489-500. doi: 10.1002/art.11229. Arthritis Rheum. 2003. PMID: 13130468
-
Proteomic surveillance of autoimmunity in osteoarthritis: identification of triosephosphate isomerase as an autoantigen in patients with osteoarthritis.Arthritis Rheum. 2004 May;50(5):1511-21. doi: 10.1002/art.20189. Arthritis Rheum. 2004. PMID: 15146421
-
[Early diagnosis of rheumatoid arthritis with a test based upon a specific antigen: cyclic citrullinated peptide].Ned Tijdschr Geneeskd. 2003 Feb 1;147(5):191-4. Ned Tijdschr Geneeskd. 2003. PMID: 12645351 Review. Dutch.
-
Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis.Immunol Rev. 2010 Jan;233(1):34-54. doi: 10.1111/j.0105-2896.2009.00850.x. Immunol Rev. 2010. PMID: 20192991 Review.
Cited by
-
Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins.Arthritis Res Ther. 2010;12(4):R132. doi: 10.1186/ar3070. Epub 2010 Jul 7. Arthritis Res Ther. 2010. PMID: 20609218 Free PMC article.
-
Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis.Arthritis Res Ther. 2008;10(4):R94. doi: 10.1186/ar2478. Epub 2008 Aug 18. Arthritis Res Ther. 2008. PMID: 18710572 Free PMC article.
-
Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis.Nat Rev Rheumatol. 2014 Nov;10(11):645-53. doi: 10.1038/nrrheum.2014.115. Epub 2014 Jul 29. Nat Rev Rheumatol. 2014. PMID: 25072264 Review.
-
An Autoantigen-ome from HS-Sultan B-Lymphoblasts Offers a Molecular Map for Investigating Autoimmune Sequelae of COVID-19.bioRxiv [Preprint]. 2021 Apr 6:2021.04.05.438500. doi: 10.1101/2021.04.05.438500. bioRxiv. 2021. PMID: 33851168 Free PMC article. Updated. Preprint.
-
Regulation of the Neurodegenerative Process Associated to Parkinson's Disease by CD4+ T-cells.J Neuroimmune Pharmacol. 2015 Dec;10(4):561-75. doi: 10.1007/s11481-015-9618-9. Epub 2015 May 28. J Neuroimmune Pharmacol. 2015. PMID: 26018603 Review.
References
-
- Tsuruha J, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Nishioka K. Implication of cartilage intermediate layer protein in cartilage destruction in subsets of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:838–845. doi: 10.1002/1529-0131(200104)44:4<838::AID-ANR140>3.0.CO;2-C. - DOI - PubMed
-
- Iwaki-Egawa S, Matsuno H, Yudoh K, Nakazawa F, Miyazaki K, Ochiai A, Hirohata S, Shimizu M, Watanabe Y. High diagnostic value of anticalpastatin autoantibodies in rheumatoid arthritis detected by ELISA using human erythrocyte calpastatin as antigen. J Rheumatol. 2004;31:17–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

