Infection of Ixodes scapularis ticks with Rickettsia monacensis expressing green fluorescent protein: a model system
- PMID: 17125789
- PMCID: PMC1868488
- DOI: 10.1016/j.jip.2006.10.003
Infection of Ixodes scapularis ticks with Rickettsia monacensis expressing green fluorescent protein: a model system
Abstract
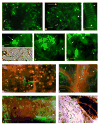
Ticks (Acari: Ixodidae) are ubiquitous hosts of rickettsiae (Rickettsiaceae: Rickettsia), obligate intracellular bacteria that occur as a continuum from nonpathogenic arthropod endosymbionts to virulent pathogens of both arthropod vectors and vertebrates. Visualization of rickettsiae in hosts has traditionally been limited to techniques utilizing fixed tissues. We report epifluorescence microscopy observations of unfixed tick tissues infected with a spotted fever group endosymbiont, Rickettsia monacensis, transformed to express green fluorescent protein (GFP). Fluorescent rickettsiae were readily visualized in tick tissues. In adult female, but not male, Ixodes scapularis infected by capillary feeding, R. monacensis disseminated from the gut and infected the salivary glands that are crucial to the role of ticks as vectors. The rickettsiae infected the respiratory tracheal system, a potential dissemination pathway and possible infection reservoir during tick molting. R. monacensis disseminated from the gut of capillary fed I. scapularis nymphs and was transstadially transmitted to adults. Larvae, infected by immersion, transstadially transmitted the rickettsiae to nymphs. Infected female I. scapularis did not transovarially transmit R. monacensis to progeny and the rickettsiae were not horizontally transmitted to a rabbit or hamsters. Survival of infected nymphal and adult I. scapularis did not differ from that of uninfected control ticks. R. monacensis did not disseminate from the gut of capillary fed adult female Amblyomma americanum (L.), or adult Dermacentor variabilis (Say) ticks of either sex. Infection of I. scapularis with R. monacensis expressing GFP provides a model system allowing visualization and study of live rickettsiae in unfixed tissues of an arthropod host.
Figures




References
-
- Adams JR, Schmidtmann ET, Azad AF. Infection of colonized fleas Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–409. - PubMed
-
- Balashov YS. Bloodsucking ticks (Ixodoidea) - vectors of diseases of man and animals. Misc Pub Ent Soc Amer. 1972;8:5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

