Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction
- PMID: 17126870
- PMCID: PMC1976290
- DOI: 10.1016/j.virol.2006.10.028
Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction
Abstract
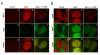
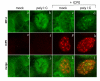
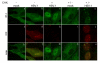
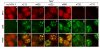
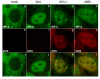
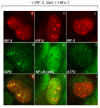
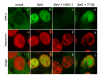
The host innate response to viral infection includes the production of interferons, which is dependent on the coordinated activity of multiple transcription factors. Herpes simplex virus 1 (HSV-1) has been shown to block efficient interferon expression by multiple mechanisms. We and others have demonstrated that HSV-1 can inhibit the transcription of genes promoted by interferon regulatory factor-3 (IRF-3), including interferon beta (IFN-beta), and that the immediate-early ICP0 protein is sufficient for this function. However, the exact mechanism by which ICP0 blocks IRF-3 activity has yet to be determined. Unlike some other viral proteins that inhibit IRF-3 activity, ICP0 does not appear to affect phosphorylation and dimerization of IRF-3. Here, we show that a portion of activated IRF-3 co-localizes with nuclear foci containing ICP0 at early times after virus infection. Co-localization to ICP0-containing foci is also seen with the IRF-3-binding partners and transcriptional co-activators, CBP and p300. In addition, using immunoprecipitation of infected cell lysates, we can immunoprecipitate a complex containing ICP0, IRF-3, and CBP. Thus we hypothesize that ICP0 recruits activated IRF-3 and CBP/p300 to nuclear structures, away from the host chromatin. This leads to the inactivation and accelerated degradation of IRF-3, resulting in reduced transcription of IFN-beta and an inhibition of the host response. Therefore, ICP0 provides an example of how viruses can block IFN-beta induction by sequestration of important transcription factors essential for the host response.
Figures






Similar articles
-
Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production.J Virol. 2004 Aug;78(16):8411-20. doi: 10.1128/JVI.78.16.8411-8420.2004. J Virol. 2004. PMID: 15280450 Free PMC article.
-
Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-κB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP.J Virol. 2013 Sep;87(17):9788-801. doi: 10.1128/JVI.01440-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824799 Free PMC article.
-
Characterization of Elements Regulating the Nuclear-to-Cytoplasmic Translocation of ICP0 in Late Herpes Simplex Virus 1 Infection.J Virol. 2018 Jan 2;92(2):e01673-17. doi: 10.1128/JVI.01673-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093084 Free PMC article.
-
Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):599-607. doi: 10.1089/jir.2009.0074. J Interferon Cytokine Res. 2009. PMID: 19694546 Review.
-
The first 30 minutes in the life of a virus: unREST in the nucleus.Cell Cycle. 2005 Aug;4(8):1019-21. doi: 10.4161/cc.4.8.1902. Epub 2005 Aug 7. Cell Cycle. 2005. PMID: 16082207 Review.
Cited by
-
Self protection from anti-viral responses--Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors.PLoS One. 2010 Jul 26;5(7):e11776. doi: 10.1371/journal.pone.0011776. PLoS One. 2010. PMID: 20668674 Free PMC article.
-
Toll-like receptors in antiviral innate immunity.J Mol Biol. 2014 Mar 20;426(6):1246-64. doi: 10.1016/j.jmb.2013.11.024. Epub 2013 Dec 3. J Mol Biol. 2014. PMID: 24316048 Free PMC article. Review.
-
C6orf106 is a novel inhibitor of the interferon-regulatory factor 3-dependent innate antiviral response.J Biol Chem. 2018 Jul 6;293(27):10561-10573. doi: 10.1074/jbc.RA117.001491. Epub 2018 May 25. J Biol Chem. 2018. PMID: 29802199 Free PMC article.
-
Role of Herpes Simplex Virus 1 γ34.5 in the Regulation of IRF3 Signaling.J Virol. 2017 Nov 14;91(23):e01156-17. doi: 10.1128/JVI.01156-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904192 Free PMC article.
-
Role of chromatin during herpesvirus infections.Biochim Biophys Acta. 2009 Jun;1790(6):456-66. doi: 10.1016/j.bbagen.2009.03.019. Epub 2009 Mar 31. Biochim Biophys Acta. 2009. PMID: 19344747 Free PMC article. Review.
References
-
- Biron CA, Sen GC. Interferons and other cytokines. In: Knipe DM, Howley PM, editors. “Fields Virology”. 4th ed. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA.: 2001. pp. 321–351.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

